Towards an Understanding of Synapse Formation
- PMID: 30359597
- PMCID: PMC6226307
- DOI: 10.1016/j.neuron.2018.09.040
Towards an Understanding of Synapse Formation
Abstract
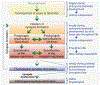
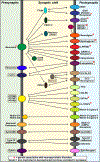
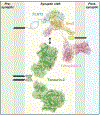
Synapses are intercellular junctions specialized for fast, point-to-point information transfer from a presynaptic neuron to a postsynaptic cell. At a synapse, a presynaptic terminal secretes neurotransmitters via a canonical release machinery, while a postsynaptic specialization senses neurotransmitters via diverse receptors. Synaptic junctions are likely organized by trans-synaptic cell-adhesion molecules (CAMs) that bidirectionally orchestrate synapse formation, restructuring, and elimination. Many candidate synaptic CAMs were described, but which CAMs are central actors and which are bystanders remains unclear. Moreover, multiple genes encoding synaptic CAMs were linked to neuropsychiatric disorders, but the mechanisms involved are unresolved. Here, I propose that engagement of multifarious synaptic CAMs produces parallel trans-synaptic signals that mediate the establishment, organization, and plasticity of synapses, thereby controlling information processing by neural circuits. Among others, this hypothesis implies that synapse formation can be understood in terms of inter- and intracellular signaling, and that neuropsychiatric disorders involve an impairment in such signaling.
Keywords: BAIs; cell-adhesion molecules; cerebellins; latrophilins; neurexins; neuroligins; synapse; synaptic plasticity; synaptogenesis; teneurins.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



References
-
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, Jin Y. (2005) The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J. Neurosci. 25, 7517–7528. - PMC - PubMed
-
- Acuna C, Liu X, Südhof TC (2016) How to Make an Active Zone: Unexpected Universal Functional Redundance between RIMs and RIM-BPs. Neuron 91, 792–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

