Development and Functional Diversification of Cortical Interneurons
- PMID: 30359598
- PMCID: PMC6290988
- DOI: 10.1016/j.neuron.2018.10.009
Development and Functional Diversification of Cortical Interneurons
Abstract
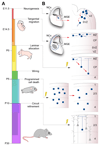
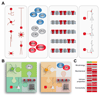
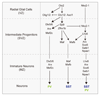
In the cerebral cortex, GABAergic interneurons have evolved as a highly heterogeneous collection of cell types that are characterized by their unique spatial and temporal capabilities to influence neuronal circuits. Current estimates suggest that up to 50 different types of GABAergic neurons may populate the cerebral cortex, all derived from progenitor cells in the subpallium, the ventral aspect of the embryonic telencephalon. In this review, we provide an overview of the mechanisms underlying the generation of the distinct types of interneurons and their integration in cortical circuits. Interneuron diversity seems to emerge through the implementation of cell-intrinsic genetic programs in progenitor cells, which unfold over a protracted period of time until interneurons acquire mature characteristics. The developmental trajectory of interneurons is also modulated by activity-dependent, non-cell-autonomous mechanisms that influence their ability to integrate in nascent circuits and sculpt their final distribution in the adult cerebral cortex.
Keywords: GABA; cerebral cortex; inhibitory; interneuron; migration; neocortex; neural circuit; neuronal identity; specification; transcription factor.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures





References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997a;278:474–476. - PubMed
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JLR. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

