The AMPA Receptor Code of Synaptic Plasticity
- PMID: 30359599
- PMCID: PMC6214363
- DOI: 10.1016/j.neuron.2018.10.018
The AMPA Receptor Code of Synaptic Plasticity
Abstract
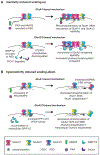
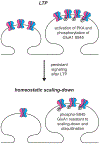
Changes in the properties and postsynaptic abundance of AMPA-type glutamate receptors (AMPARs) are major mechanisms underlying various forms of synaptic plasticity, including long-term potentiation (LTP), long-term depression (LTD), and homeostatic scaling. The function and the trafficking of AMPARs to and from synapses is modulated by specific AMPAR GluA1-GluA4 subunits, subunit-specific protein interactors, auxiliary subunits, and posttranslational modifications. Layers of regulation are added to AMPAR tetramers through these different interactions and modifications, increasing the computational power of synapses. Here we review the reliance of synaptic plasticity on AMPAR variants and propose "the AMPAR code" as a conceptual framework. The AMPAR code suggests that AMPAR variants will be predictive of the types and extent of synaptic plasticity that can occur and that a hierarchy exists such that certain AMPARs will be disproportionally recruited to synapses during LTP/homeostatic scaling up, or removed during LTD/homeostatic scaling down.
Keywords: NMDA receptors; glutamate receptors; homeostatic plasticity; homeostatic scaling; learning; long-term depression; long-term potentiation; memory; synaptic plasticity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- ALBERI S, BODA B, STEINER P, NIKONENKO I, HIRLING H & MULLER D 2005. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol Cell Neurosci, 29, 313–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

