The Meningeal Lymphatic System: A New Player in Neurophysiology
- PMID: 30359603
- PMCID: PMC6268162
- DOI: 10.1016/j.neuron.2018.09.022
The Meningeal Lymphatic System: A New Player in Neurophysiology
Abstract
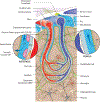
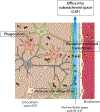
The nature of fluid dynamics within the brain parenchyma is a focus of intensive research. Of particular relevance is its participation in diseases associated with protein accumulation and aggregation in the brain, such as Alzheimer's disease (AD). The meningeal lymphatic vessels have recently been recognized as an important player in the complex circulation and exchange of soluble contents between the cerebrospinal fluid (CSF) and the interstitial fluid (ISF). In aging mammals, for example, impaired functioning of the meningeal lymphatic vessels can lead to accelerated accumulation of toxic amyloid beta protein in the brain parenchyma, thus aggravating AD-related pathology. Given that meningeal lymphatic vessels are functionally linked to paravascular influx/efflux of the CSF/ISF, and in light of recent findings that certain cytokines, classically perceived as immune molecules, exert neuromodulatory effects, it is reasonable to suggest that the activity of meningeal lymphatics could alter the accessibility of CSF-borne immune neuromodulators to the brain parenchyma, thereby altering their effects on the brain. Accordingly, in this Perspective we propose that the meningeal lymphatic system can be viewed as a novel player in neurophysiology.
Keywords: Alzheimer’s disease; cerebrospinal fluid; cytokines; glia; glymphatic; immune cells; interstitial fluid; lymphatic vessels; meninges; neuromodulation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
J.K. is an Advisor to PureTech Health/Ariya.
Figures


References
-
- Agorastos A, Hauger RL, Barkauskas DA, Moeller-Bertram T, Clopton PL, Haji U, Lohr JB, Geracioti TD Jr., Patel PM, Chrousos GP, and Baker DG (2014). Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology 44, 71–82. - PubMed
-
- Alitalo K (2011). The lymphatic vasculature in disease. Nat Med 17, 1371–1380. - PubMed
-
- Alitalo K, Tammela T, and Petrova TV (2005). Lymphangiogenesis in development and human disease. Nature 438, 946–953. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

