Lost in Translation: Traversing the Complex Path from Genomics to Therapeutics in Autism Spectrum Disorder
- PMID: 30359605
- PMCID: PMC6989093
- DOI: 10.1016/j.neuron.2018.10.015
Lost in Translation: Traversing the Complex Path from Genomics to Therapeutics in Autism Spectrum Disorder
Abstract
Recent progress in the genomics of non-syndromic autism spectrum disorder (nsASD) highlights rare, large-effect, germline, heterozygous de novo coding mutations. This distinguishes nsASD from later-onset psychiatric disorders where gene discovery efforts have predominantly yielded common alleles of small effect. These differences point to distinctive opportunities for clarifying the neurobiology of nsASD and developing novel treatments. We argue that the path ahead also presents key challenges, including distinguishing human pathophysiology from the potentially pleiotropic neurobiology mediated by established risk genes. We present our view of some of the conceptual limitations of traditional studies of model organisms, suggest a strategy focused on investigating the convergence of multiple nsASD genes, and propose that the detailed characterization of the molecular and cellular landscapes of developing human brain is essential to illuminate disease mechanisms. Finally, we address how recent advances are leading to novel strategies for therapeutics that target various points along the path from genes to behavior.
Keywords: autism spectrum disorder; convergence; convergence neuroscience; de novo mutation; gene therapy; genomics; human brain development; neurodevelopmental disorders; non-syndromic autism spectrum disorder; transcriptomics.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
Matthew W. State serves on the scientific advisory boards and has stock or stock options for BlackThorn therapeutics and ARett pharmaceuticals.
Figures
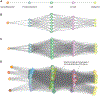


References
Publication types
MeSH terms
Grants and funding
- R01 MH110928/MH/NIMH NIH HHS/United States
- U01 MH105575/MH/NIMH NIH HHS/United States
- U01 MH115747/MH/NIMH NIH HHS/United States
- R01 MH102342/MH/NIMH NIH HHS/United States
- R01 MH109901/MH/NIMH NIH HHS/United States
- U01 MH111662/MH/NIMH NIH HHS/United States
- R01 MH109904/MH/NIMH NIH HHS/United States
- U01 MH116487/MH/NIMH NIH HHS/United States
- U01 MH106874/MH/NIMH NIH HHS/United States
- R01 MH110926/MH/NIMH NIH HHS/United States
- U01 MH116488/MH/NIMH NIH HHS/United States
- P50 MH106934/MH/NIMH NIH HHS/United States

