Trypanothione synthetase confers growth, survival advantage and resistance to anti-protozoal drugs in Trypanosoma cruzi
- PMID: 30359758
- PMCID: PMC6331241
- DOI: 10.1016/j.freeradbiomed.2018.10.436
Trypanothione synthetase confers growth, survival advantage and resistance to anti-protozoal drugs in Trypanosoma cruzi
Abstract
Background: Chagas cardiomyopathy, caused by Trypanosoma cruzi infection, continues to be a neglected illness, and has a major impact on global health. The parasite undergoes several stages of morphological and biochemical changes during its life cycle, and utilizes an elaborated antioxidant network to overcome the oxidants barrier and establish infection in vector and mammalian hosts. Trypanothione synthetase (TryS) catalyzes the biosynthesis of glutathione-spermidine adduct trypanothione (T(SH)2) that is the principal intracellular thiol-redox metabolite in trypanosomatids.
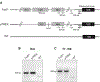
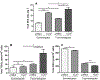
Methods and results: We utilized genetic overexpression (TryShi) and pharmacological inhibition approaches to examine the role of TryS in T. cruzi proliferation, tolerance to oxidative stress and resistance to anti-protozoal drugs. Our data showed the expression and activity of TryS was increased in all morphological stages of TryShi (vs. control) parasites. In comparison to controls, the TryShi epimastigotes (insect stage) recorded shorter doubling time, and both epimastigotes and infective trypomastigotes of TryShi exhibited 36-71% higher resistance to H2O2 (50-1000 μM) and heavy metal (1-500 μM) toxicity. Treatment with TryS inhibitors (5-30 μM) abolished the proliferation and survival advantages against H2O2 pressure in a dose-dependent manner in both TryShi and control parasites. Further, epimastigote and trypomastigote forms of TryShi (vs. control) T. cruzi tolerated higher doses of benznidazole and nifurtimox, the drugs currently administered for acute Chagas disease treatment.
Conclusions: TryS is essential for proliferation and survival of T. cruzi under normal and oxidant stress conditions, and provides an advantage to the parasite to develop resistance against currently used anti-trypanosomal drugs. TryS indispensability has been chemically validated with inhibitors that may be useful for drug combination therapy against Chagas disease.
Keywords: Anti-parasite drugs; Chagas disease; Paullones; Small molecule inhibitors; Trypanosoma cruzi; Trypanothione synthetase.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest:
The authors declare no financial conflict of interest.
Figures
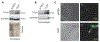

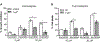


References
-
- WHO | Chagas disease (American trypanosomiasis), (2017). http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed April 15, 2018).
-
- Lauria-Pires L, Braga MS, Vexenat AC, Nitz N, Simões-Barbosa A, Tinoco DL, Teixeira ARL, Progressive chronic chagas heart disease ten years after treatment with antiTrypanosoma cruzi nitroderivatives, Am. J. Trop. Med. Hyg 63 (2000) 111–118. - PubMed
-
- Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy, N. Engl. J. Med 373 (2015) 1295–1306. doi:10.1056/NEJMoa1507574. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

