Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains
- PMID: 30360435
- PMCID: PMC6211117
- DOI: 10.3390/ijerph15102321
Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains
Abstract
There is a growing body of evidence that flavonoids show antibacterial activity against both Gram-positive and Gram-negative bacteria. The mechanisms of action of phenolic compounds on bacterial cell have been partially attributed to damage to the bacterial membrane, inhibition of virulence factors such as enzymes and toxins, and suppression of bacterial biofilm formation. What is more, some natural polyphenols, aside from direct antibacterial activity, exert a synergistic effect when combined with common chemotherapeutics. Many studies have proved that in synergy with antibiotics plant flavonoids pose a promising alternative for therapeutic strategies against drug resistant bacteria. In this review most recent reports on antimicrobial action of polyphenols on Staphylococcus aureus strains are described, highlighting where proven, the mechanisms of action and the structure⁻activity relationships. Since many reports in this field are, to some extent, conflicting, a unified in vitro and in vivo susceptibility testing algorithms should be introduced to ensure the selection of effective antibacterial polyphenolic compounds with low cytotoxicity and minimal side effects.
Keywords: Staphylococcus aureus; antibacterial activity; antibiotics; polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
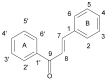


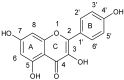
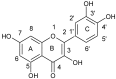
References
-
- Qin R., Xiao K., Li B., Jiang W., Peng W., Zheng J., Zhou H. The combination of catechin and epicatechin gallate from Fructus Crataegi potentiates beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Int. J. Mol. Sci. 2013;14:1802–1821. doi: 10.3390/ijms14011802. - DOI - PMC - PubMed
-
- Miklasińska M., Kępa M., Wojtyczka R.D., Idzik D., Zdebik A., Orlewska K., Wąsik T.J. Antibacterial activity of protocatechuic acid ethyl ester on Staphylococcus aureus clinical strains alone and in combination with antistaphylococcal drugs. Molecules. 2015;20:13536–13549. doi: 10.3390/molecules200813536. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

