In Silico Studies on Compounds Derived from Calceolaria: Phenylethanoid Glycosides as Potential Multitarget Inhibitors for the Development of Pesticides
- PMID: 30360548
- PMCID: PMC6322355
- DOI: 10.3390/biom8040121
In Silico Studies on Compounds Derived from Calceolaria: Phenylethanoid Glycosides as Potential Multitarget Inhibitors for the Development of Pesticides
Abstract
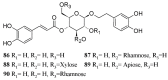
An increasing occurrence of resistance in insect pests and high mammal toxicity exhibited by common pesticides increase the need for new alternative molecules. Among these alternatives, bioinsecticides are considered to be environmentally friendly and safer than synthetic insecticides. Particularly, plant extracts have shown great potential in laboratory conditions. However, the lack of studies that confirm their mechanisms of action diminishes their potential applications on a large scale. Previously, we have reported the insect growth regulator and insecticidal activities of secondary metabolites isolated from plants of the Calceolaria genus. Herein, we report an in silico study of compounds isolated from Calceolaria against acetylcholinesterase, prophenoloxidase, and ecdysone receptor. The molecular docking results are consistent with the previously reported experimental results, which were obtained during the bioevaluation of Calceolaria extracts. Among the compounds, phenylethanoid glycosides, such as verbascoside, exhibited good theoretical affinity to all the analyzed targets. In light of these results, we developed an index to evaluate potential multitarget insecticides based on docking scores.
Keywords: Calceolaria; bioinsecticides; molecular docking; multitarget; phenylethanoid glycosides; structure–activity relationship.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Panagiotakopulu E., Buckland P.C., Day P.M. Natural Insecticides and Insect Repellents in Antiquity: A Review of the Evidence. J. Archeol. Sci. 1995;22:705–710. doi: 10.1016/S0305-4403(95)80156-1. - DOI
-
- Sporleder M., Lacey L.A. Insect Pests of Potato. Elsevier; Amsterdam, The Netherlands: 2013. Biopesticides; pp. 463–497.
-
- Isman M.B. Bridging the gap: Moving botanical insecticides from the laboratory to the farm. Ind. Crops Prod. 2017;110:10–14. doi: 10.1016/j.indcrop.2017.07.012. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

