Appraising of the Clinical Practice Guidelines Quality in the Non-Pharmacological Management of Chemotherapy-Induced Febrile Neutropenia; A Review
- PMID: 30360594
- PMCID: PMC6291058
- DOI: 10.22034/APJCP.2018.19.10.2701
Appraising of the Clinical Practice Guidelines Quality in the Non-Pharmacological Management of Chemotherapy-Induced Febrile Neutropenia; A Review
Abstract
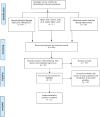
Background: Febrile neutropenia is a common and serious chemotherapy side effect, is associated with increased mortality, morbidity, and treatment expenditures. Several CPGs (Clinical practice guidelines) have been released for managing chemotherapy-induced febrile neutropenia. The aim of this study is Appraisal of the clinical practice Guidelines quality in the management of chemotherapy-induced febrile neutropenia. Methods: A review study with a systematic search of the present CPGs for the management of chemotherapy-induced febrile neutropenia. After screening the CPGs based on eligibility criteria, three CPGs were selected and 5 independent reviewers appraised them for methodological quality by using the AGREE II Instrument. Results: Three CPGs were included; all of them were evidence-based guidelines. The clarity of presentation domain scored the highest and the applicability domain has the lowest score among all domains of AGREE instrument and the rest of domains scored as descending respectively; Scope and purpose, stakeholder involvement, editorial independence, rigor of development. In general, the intra-class correlation coefficient (ICC) scores of all domains were very good according to the Landis and Koch’s scale, except the Applicability domain scored as substantial. Conclusions: This study showed the quality of appraised CPGs. Three domains of these CPGs based on the AGREE instrument scored less than other domains and were in relatively unfavorable status: applicability, rigor of development, editorial independence. Given the importance of these domains in guideline implementation, it is necessary to take actions for reducing these defects.
Keywords: Quality; clinical practice guidelines; AGREE II Instrument; chemotherapy; induced febrile neutropenia.
Creative Commons Attribution License
Figures
Similar articles
-
Critical appraisal of clinical practice guidelines for adult cancer patients with febrile neutropenia.Int J Pharm Pract. 2018 Feb;26(1):49-54. doi: 10.1111/ijpp.12357. Epub 2017 Mar 27. Int J Pharm Pract. 2018. PMID: 28349577
-
A systematic review of the quality of clinical practice guidelines for lymphedema, as assessed using the Appraisal of Guidelines for Research and Evaluation II instrument.J Vasc Surg Venous Lymphat Disord. 2020 Jul;8(4):685-692. doi: 10.1016/j.jvsv.2020.04.008. Epub 2020 Apr 23. J Vasc Surg Venous Lymphat Disord. 2020. PMID: 32335331
-
A Systematic Critical Appraisal of Clinical Practice Guidelines in Juvenile Idiopathic Arthritis Using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) Instrument.PLoS One. 2015 Sep 10;10(9):e0137180. doi: 10.1371/journal.pone.0137180. eCollection 2015. PLoS One. 2015. PMID: 26356098 Free PMC article.
-
Guidelines in Low and Middle Income Countries Paper 3: Appraisal of Philippine Clinical Practice Guidelines using Appraisal of Guidelines for Research and Evaluation II: improvement needed for rigor, applicability, and editorial independence.J Clin Epidemiol. 2020 Nov;127:184-190. doi: 10.1016/j.jclinepi.2020.06.036. Epub 2020 Jul 1. J Clin Epidemiol. 2020. PMID: 32621853
-
Critical Appraisal of International Clinical Practice Guidelines in Kidney Transplantation Using the Appraisal of Guidelines for Research and Education II Tool: A Systematic Review.Transplantation. 2018 Sep;102(9):1419-1439. doi: 10.1097/TP.0000000000002255. Transplantation. 2018. PMID: 30124634
References
-
- Armstrong JJ, Rodrigues IB, Wasiuta T, et al. Quality assessment of osteoporosis clinical practice guidelines for physical activity and safe movement: An agree ii appraisal. Arch Osteoporos. 2016;11:1–10. - PubMed
-
- Brouwers M, Kho ME, Browman GP, et al. On behalf of the agree next steps consortium. Agree ii: Advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2010b;182:839–42.