Electrical impedance tomography in acute respiratory distress syndrome
- PMID: 30360753
- PMCID: PMC6203288
- DOI: 10.1186/s13054-018-2195-6
Electrical impedance tomography in acute respiratory distress syndrome
Abstract
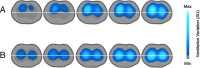
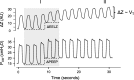
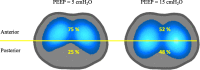
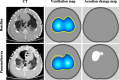
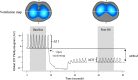
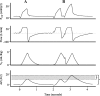
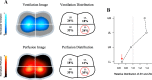
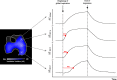
Acute respiratory distress syndrome (ARDS) is a clinical entity that acutely affects the lung parenchyma, and is characterized by diffuse alveolar damage and increased pulmonary vascular permeability. Currently, computed tomography (CT) is commonly used for classifying and prognosticating ARDS. However, performing this examination in critically ill patients is complex, due to the need to transfer these patients to the CT room. Fortunately, new technologies have been developed that allow the monitoring of patients at the bedside. Electrical impedance tomography (EIT) is a monitoring tool that allows one to evaluate at the bedside the distribution of pulmonary ventilation continuously, in real time, and which has proven to be useful in optimizing mechanical ventilation parameters in critically ill patients. Several clinical applications of EIT have been developed during the last years and the technique has been generating increasing interest among researchers. However, among clinicians, there is still a lack of knowledge regarding the technical principles of EIT and potential applications in ARDS patients. The aim of this review is to present the characteristics, technical concepts, and clinical applications of EIT, which may allow better monitoring of lung function during ARDS.
Keywords: Acute respiratory distress syndrome; Electrical impedance tomography; Lung imaging; Mechanical ventilation; Ventilation distribution.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from the patient.
Competing interests
EC, CM, and JBB report personal fees from Timpel S.A. during the conduct of the study. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Herridge Margaret S., Cheung Angela M., Tansey Catherine M., Matte-Martyn Andrea, Diaz-Granados Natalia, Al-Saidi Fatma, Cooper Andrew B., Guest Cameron B., Mazer C. David, Mehta Sangeeta, Stewart Thomas E., Barr Aiala, Cook Deborah, Slutsky Arthur S. One-Year Outcomes in Survivors of the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. - DOI - PubMed

