Cortical Actin Dynamics in Endothelial Permeability
- PMID: 30360779
- PMCID: PMC6263148
- DOI: 10.1016/bs.ctm.2018.09.003
Cortical Actin Dynamics in Endothelial Permeability
Abstract
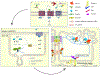
The pulmonary endothelial cell forms a critical semi-permeable barrier between the vascular and interstitial space. As part of the blood-gas barrier in the lung, the endothelium plays a key role in normal physiologic function and pathologic disease. Changes in endothelial cell shape, defined by its plasma membrane, determine barrier integrity. A number of key cytoskeletal regulatory and effector proteins including non-muscle myosin light chain kinase, cortactin, and Arp 2/3 mediate actin rearrangements to form cortical and membrane associated structures in response to barrier enhancing stimuli. These actin formations support and interact with junctional complexes and exert forces to protrude the lipid membrane to and close gaps between individual cells. The current knowledge of these cytoskeletal processes and regulatory proteins are the subject of this review. In addition, we explore novel advancements in cellular imaging that are poised to shed light on the complex nature of pulmonary endothelial permeability.
Keywords: ARDS; Arp 2/3; Atomic force microscopy; Cortactin; Cortical actin; Endothelial permeability; Intravital microscopy; Lamellipodia; Lung injury; Non-muscle myosin light chain kinase; Super-resolution microscopy.
© 2018 Elsevier Inc. All rights reserved.
Figures

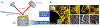
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

