Monocyte and Macrophage Dynamics in the Cardiovascular System: JACC Macrophage in CVD Series (Part 3)
- PMID: 30360828
- PMCID: PMC6258199
- DOI: 10.1016/j.jacc.2018.08.2150
Monocyte and Macrophage Dynamics in the Cardiovascular System: JACC Macrophage in CVD Series (Part 3)
Abstract
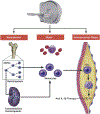
It has long been recognized that the bone marrow is the primary site of origin for circulating monocytes that may later become macrophages in atherosclerotic lesions. However, only in recent times has the complex relationship among the bone marrow, monocytes/macrophages, and atherosclerotic plaques begun to be understood. Moreover, the systemic nature of these interactions, which also involves additional compartments such as extramedullary hematopoietic sites (i.e., spleen), is only just becoming apparent. In parallel, progressive advances in imaging and cell labeling techniques have opened new opportunities for in vivo imaging of monocyte/macrophage trafficking in atherosclerotic lesions and at the systemic level. In this Part 3 of a 4-part review series covering the macrophage in cardiovascular disease, the authors intersect systemic biology with advanced imaging techniques to explore monocyte and macrophage dynamics in the cardiovascular system, with an emphasis on how events at the systemic level might affect local atherosclerotic plaque biology.
Keywords: atherosclerosis; bone marrow; cardiovascular; imaging; macrophage.
Copyright © 2018 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures:
The authors declare no conflicts of interest.
Figures


References
-
- Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 2012;366:54–63. - PubMed
-
- Bloom D, Cafiero E, Jané-Llopis E. The Global Economic Burden of Non-communicable Diseases Cambridge, MA: Harvard School of Public Health, 2011.
-
- Ridker PM, Everett BM, Thuren T et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119–1131. - PubMed
-
- Ridker PM, MacFadyen JG, Thuren T et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

