Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study
- PMID: 30361119
- PMCID: PMC7045907
- DOI: 10.1016/S2213-2600(18)30344-8
Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study
Erratum in
-
Correction to Lancet Respir Med 2019; 7: 115-28.Lancet Respir Med. 2019 Feb;7(2):e9. doi: 10.1016/S2213-2600(18)30475-2. Epub 2018 Nov 13. Lancet Respir Med. 2019. PMID: 30446467 No abstract available.
-
Correction to Lancet Respir Med 2019; 7: 115-28.Lancet Respir Med. 2019 Mar;7(3):e12. doi: 10.1016/S2213-2600(19)30032-3. Lancet Respir Med. 2019. PMID: 30823977 No abstract available.
Abstract
Background: Paediatric acute respiratory distress syndrome (PARDS) is associated with high mortality in children, but until recently no paediatric-specific diagnostic criteria existed. The Pediatric Acute Lung Injury Consensus Conference (PALICC) definition was developed to overcome limitations of the Berlin definition, which was designed and validated for adults. We aimed to determine the incidence and outcomes of children who meet the PALICC definition of PARDS.
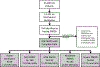
Methods: In this international, prospective, cross-sectional, observational study, 145 paediatric intensive care units (PICUs) from 27 countries were recruited, and over a continuous 5 day period across 10 weeks all patients were screened for enrolment. Patients were included if they had a new diagnosis of PARDS that met PALICC criteria during the study week. Exclusion criteria included meeting PARDS criteria more than 24 h before screening, cyanotic heart disease, active perinatal lung disease, and preparation or recovery from a cardiac intervention. Data were collected on the PICU characteristics, patient demographics, and elements of PARDS (ie, PARDS risk factors, hypoxaemia severity metrics, type of ventilation), comorbidities, chest imaging, arterial blood gas measurements, and pulse oximetry. The primary outcome was PICU mortality. Secondary outcomes included 90 day mortality, duration of invasive mechanical and non-invasive ventilation, and cause of death.
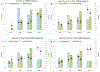
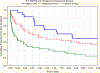
Findings: Between May 9, 2016, and June 16, 2017, during the 10 study weeks, 23 280 patients were admitted to participating PICUs, of whom 744 (3·2%) were identified as having PARDS. 95% (708 of 744) of patients had complete data for analysis, with 17% (121 of 708; 95% CI 14-20) mortality, whereas only 32% (230 of 708) of patients met Berlin criteria with 27% (61 of 230) mortality. Based on hypoxaemia severity at PARDS diagnosis, mortality was similar among those who were non-invasively ventilated and with mild or moderate PARDS (10-15%), but higher for those with severe PARDS (33% [54 of 165; 95% CI 26-41]). 50% (80 of 160) of non-invasively ventilated patients with PARDS were subsequently intubated, with 25% (20 of 80; 95% CI 16-36) mortality. By use of PALICC PARDS definition, severity of PARDS at 6 h after initial diagnosis (area under the curve [AUC] 0·69, 95% CI 0·62-0·76) discriminates PICU mortality better than severity at PARDS diagnosis (AUC 0·64, 0·58-0·71), and outperforms Berlin severity groups at 6 h (0·64, 0·58-0·70; p=0·01).
Interpretation: The PALICC definition identified more children as having PARDS than the Berlin definition, and PALICC PARDS severity groupings improved the stratification of mortality risk, particularly when applied 6 h after PARDS diagnosis. The PALICC PARDS framework should be considered for use in future epidemiological and therapeutic research among children with PARDS.
Funding: University of Southern California Clinical Translational Science Institute, Sainte Justine Children's Hospital, University of Montreal, Canada, Réseau en Santé Respiratoire du Fonds de Recherche Quebec-Santé, and Children's Hospital Los Angeles, Department of Anesthesiology and Critical Care Medicine.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests
Dr. Khemani reports grants from University of Southern California CTSI, grants and non-financial support from Children’s Hospital Los Angeles, Department of Anesthesiology and Critical Care, during the conduct of the study; grants from National Institutes of Health, personal fees from OrangeMed, personal fees from Hamilton Medical, outside the submitted work; .Dr. Jouvet reports grants from CHU- Sainte Justine, University of Montreal Canada, grants from Reseau en Sante Respiratoire du Fonds de Recherche Quebec-Sante (FRQS), grants and non-financial support from Air Liquide Sante, outside the submitted work; Dr. Yehya reports grants from NIH, during the conduct of the study; Dr. Thomas reports personal fees from Therabron, personal fees from CareFusion, grants from Gene Fluidics, outside the submitted work; Dr. Newth reports personal fees from Philips Research North America, outside the submitted work; All other authors declared no conflicts of interest.
Figures

Comment in
-
Personalising care of acute respiratory distress syndrome according to patients' age.Lancet Respir Med. 2019 Feb;7(2):100-101. doi: 10.1016/S2213-2600(18)30429-6. Epub 2018 Oct 22. Lancet Respir Med. 2019. PMID: 30361118 No abstract available.
References
-
- Schouten LR, Veltkamp F, Bos AP, et al. Incidence and Mortality of Acute Respiratory Distress Syndrome in Children: A Systematic Review and Meta-Analysis. Critical care medicine. 2016;44(4):819–829. - PubMed
-
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994; 149:(3)818–24.. - PubMed
-
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Medicine. 2012;38(10):10. - PubMed
-
- Khemani RG, Smith LS, Zimmerman JJ, Erickson S, PALICC Group. Pediatric Acute Respiratory Distress Syndrome: Definition, Incidence, and Epidemiology: Proceedings From the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine. 2015;16(5_suppl):S23–S40 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

