The Receptor-like Pseudokinase GHR1 Is Required for Stomatal Closure
- PMID: 30361234
- PMCID: PMC6305979
- DOI: 10.1105/tpc.18.00441
The Receptor-like Pseudokinase GHR1 Is Required for Stomatal Closure
Abstract
Guard cells control the aperture of stomatal pores to balance photosynthetic carbon dioxide uptake with evaporative water loss. Stomatal closure is triggered by several stimuli that initiate complex signaling networks to govern the activity of ion channels. Activation of SLOW ANION CHANNEL1 (SLAC1) is central to the process of stomatal closure and requires the leucine-rich repeat receptor-like kinase (LRR-RLK) GUARD CELL HYDROGEN PEROXIDE-RESISTANT1 (GHR1), among other signaling components. Here, based on functional analysis of nine Arabidopsis thaliana ghr1 mutant alleles identified in two independent forward-genetic ozone-sensitivity screens, we found that GHR1 is required for stomatal responses to apoplastic reactive oxygen species, abscisic acid, high CO2 concentrations, and diurnal light/dark transitions. Furthermore, we show that the amino acid residues of GHR1 involved in ATP binding are not required for stomatal closure in Arabidopsis or the activation of SLAC1 anion currents in Xenopus laevis oocytes and present supporting in silico and in vitro evidence suggesting that GHR1 is an inactive pseudokinase. Biochemical analyses suggested that GHR1-mediated activation of SLAC1 occurs via interacting proteins and that CALCIUM-DEPENDENT PROTEIN KINASE3 interacts with GHR1. We propose that GHR1 acts in stomatal closure as a scaffolding component.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures
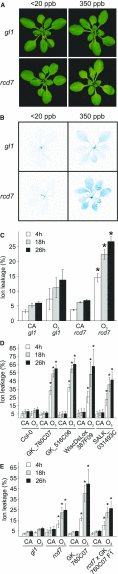
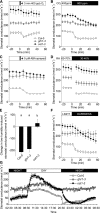

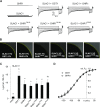
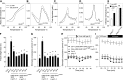





Comment in
-
Active Support: GHR1 Is a Pseudokinase That Acts as a Scaffolding Component.Plant Cell. 2018 Nov;30(11):2648. doi: 10.1105/tpc.18.00810. Epub 2018 Oct 29. Plant Cell. 2018. PMID: 30373758 Free PMC article. No abstract available.
References
-
- Bauer H., Ache P., Lautner S., Fromm J., Hartung W., Al-Rasheid K.A., Sonnewald S., Sonnewald U., Kneitz S., Lachmann N., Mendel R.R., Bittner F., et al. (2013). The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 23: 53–57. - PubMed
-
- Bent A. (2006). Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 343: 87–103. - PubMed
-
- Blaum B.S., Mazzotta S., Nöldeke E.R., Halter T., Madlung J., Kemmerling B., Stehle T. (2014). Structure of the pseudokinase domain of BIR2, a regulator of BAK1-mediated immune signaling in Arabidopsis. J. Struct. Biol. 186: 112–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

