Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia
- PMID: 30361262
- PMCID: PMC6318429
- DOI: 10.1182/blood-2018-08-868752
Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia
Abstract
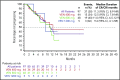
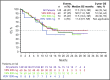
Older patients with acute myeloid leukemia (AML) respond poorly to standard induction therapy. B-cell lymphoma 2 (BCL-2) overexpression is implicated in survival of AML cells and treatment resistance. We report safety and efficacy of venetoclax with decitabine or azacitidine from a large, multicenter, phase 1b dose-escalation and expansion study. Patients (N = 145) were at least 65 years old with treatment-naive AML and were ineligible for intensive chemotherapy. During dose escalation, oral venetoclax was administered at 400, 800, or 1200 mg daily in combination with either decitabine (20 mg/m2, days 1-5, intravenously [IV]) or azacitidine (75 mg/m2, days 1-7, IV or subcutaneously). In the expansion, 400 or 800 mg venetoclax with either hypomethylating agent (HMA) was given. Median age was 74 years, with poor-risk cytogenetics in 49% of patients. Common adverse events (>30%) included nausea, diarrhea, constipation, febrile neutropenia, fatigue, hypokalemia, decreased appetite, and decreased white blood cell count. No tumor lysis syndrome was observed. With a median time on study of 8.9 months, 67% of patients (all doses) achieved complete remission (CR) + CR with incomplete count recovery (CRi), with a CR + CRi rate of 73% in the venetoclax 400 mg + HMA cohort. Patients with poor-risk cytogenetics and those at least 75 years old had CR + CRi rates of 60% and 65%, respectively. The median duration of CR + CRi (all patients) was 11.3 months, and median overall survival (mOS) was 17.5 months; mOS has not been reached for the 400-mg venetoclax cohort. The novel combination of venetoclax with decitabine or azacitidine was effective and well tolerated in elderly patients with AML (This trial was registered at www.clinicaltrials.gov as #NCT02203773).
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.D.D. received research funding from AbbVie/Genentech, Agios, Celgene, Daiichi Sankyo, Millennium, and Novartis and served as consultant for Agios, and an advisory board member for AbbVie, Agios, Bayer, Celgene, Karyopharm, and Medimmune; K.P. received research funding from AbbVie, Agios, Daiichi Sankyo, and Millennium and is an advisory board member for AbbVie, Astellas, and Boston BioMedical; B.A.J. served as consultant for AbbVie, Amgen, and Tolero and as an advisory board member for Celgene and received research funding to his institution from AbbVie, Incyte, Forma, Celgene, Daiichi Sankyo, Pharmacyclics, Genentech/Roche, Glycomimetics, AROG, Accelerated Medical Diagnostics, LP Therapeutics, Esanex, and Kalobios; M.A. received research funding from Cephalon Oncology; P.S.B. received research funding from AbbVie, Bristol-Myers Squibb, Glycomimetics, JW Pharmaceutical, Amgen, Novartis, Trovagene, Trethera, and Aptose and served as an advisory board member for Pfizer and CVS Caremark; O.F. served on an advisory board and speakers’ bureau for Celgene, Aggios, and Jazz and as n advisory board member for AbbVie; M.K. served as a consultant for AbbVie, Genentech, and F. Hoffman La-Roche and as an advisory board member for F. Hoffman La-Roche and holds shares from Reata Pharmaceuticals and received honoraria from Amgen, Abbvie, and Genentech and research funding from AbbVie, Genentech, Eli Lilly, Cellectis, Calithera, Stemline, Threshold, Flexus Biosciences, Novartis, Ablynx, and Agios; A.H.W. served in an advisory role for AbbVie, Celgene, Novartis, Amgen, and Servier and received research funding from AbbVie, Celgene, and Servier and honoraria from AbbVie, Celgene, Novartis, Amgen, and Servier; H.M.K. received honoraria from AbbVie, Actinium, Agios, Amgen, Immunogen, Orsinex, Pfizer, and Takeda and research grants from AbbVie, Agios, Amgen, Ariad, Astex, Bristol-Myers Squibb, Cyclacel, Immunogen, Jazz, and Pfizer; T.X. is an employee of AbbVie and may hold stock or stock options; W.-J.H. is an employee of Genentech and may hold stock or stock options; B.C. is an employee of AbbVie and may hold stock or stock options; J.P. is an employee of AbbVie and may hold stock or stock options; D.A.P. serves on the data safety monitoring board for GlycoMimetics and as an advisory board member for Pfizer, Jazz, Takeda, Curis, Argenx, Agios, Servier, Celgene, and AbbVie and received research funding from AbbVie, Celgene, and Pfizer; and A.L. serves as a consultant for and receives research funding from AbbVie, TetraLogic, AstraZeneca, and Novartis and is a cofounder of Flash Therapeutics and Vivid Bioscience. V.P. declares no competing financial interests.
Figures

Comment in
-
Venetoclax in AML: aiming for "just right".Blood. 2019 Jan 3;133(1):3-4. doi: 10.1182/blood-2018-11-883454. Blood. 2019. PMID: 30606807 No abstract available.
-
Harnessing the Therapeutic Value of Venetoclax: A Breakthrough Therapy in Acute Myeloid Leukemia.J Clin Oncol. 2021 Sep 1;39(25):2742-2748. doi: 10.1200/JCO.21.00080. Epub 2021 Jun 4. J Clin Oncol. 2021. PMID: 34086506 Free PMC article. No abstract available.
References
-
- Kantarjian H, O’brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090-1098. - PubMed

