Macrophage TNF-α licenses donor T cells in murine bone marrow failure and can be implicated in human aplastic anemia
- PMID: 30361263
- PMCID: PMC6307988
- DOI: 10.1182/blood-2018-05-844928
Macrophage TNF-α licenses donor T cells in murine bone marrow failure and can be implicated in human aplastic anemia
Abstract
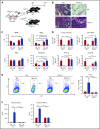
Interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) have been implicated historically in the immune pathophysiology of aplastic anemia (AA) and other bone marrow (BM) failure syndromes. We recently defined the essential roles of IFN-γ produced by donor T cells and the IFN-γ receptor in the host in murine immune-mediated BM failure models. TNF-α has been assumed to function similarly to IFN-γ. We used our murine models and mice genetically deficient in TNF-α or TNF-α receptors (TNF-αRs) to establish an analogous mechanism. Unexpectedly, infusion of TNF-α-/- donor lymph node (LN) cells into CByB6F1 recipients or injection of FVB LN cells into TNF-αR-/- recipients both induced BM failure, with concurrent marked increases in plasma IFN-γ and TNF-α levels. Surprisingly, in TNF-α-/- recipients, BM damage was attenuated, suggesting that TNF-α of host origin was essential for immune destruction of hematopoiesis. Depletion of host macrophages before LN injection reduced T-cell IFN-γ levels and reduced BM damage, whereas injection of recombinant TNF-α into FVB-LN cell-infused TNF-α-/- recipients increased T-cell IFN-γ expression and accelerated BM damage. Furthermore, infusion of TNF-αR-/- donor LN cells into CByB6F1 recipients reduced BM T-cell infiltration, suppressed T-cell IFN-γ production, and alleviated BM destruction. Thus, TNF-α from host macrophages and TNF-αR expressed on donor effector T cells were critical in the pathogenesis of murine immune-mediated BM failure, acting by modulation of IFN-γ secretion. In AA patients, TNF-α-producing macrophages in the BM were more frequent than in healthy controls, suggesting the involvement of this cytokine and these cells in human disease.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures






References
-
- Zoumbos NC, Djeu JY, Young NS. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J Immunol. 1984;133(2):769-774. - PubMed
-
- Broxmeyer HE, Cooper S, Rubin BY, Taylor MW. The synergistic influence of human interferon-gamma and interferon-alpha on suppression of hematopoietic progenitor cells is additive with the enhanced sensitivity of these cells to inhibition by interferons at low oxygen tension in vitro. J Immunol. 1985;135(4):2502-2506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

