Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway
- PMID: 30361437
- PMCID: PMC6314125
- DOI: 10.1074/jbc.RA118.004434
Oligomerization of Frizzled and LRP5/6 protein initiates intracellular signaling for the canonical WNT/β-catenin pathway
Abstract
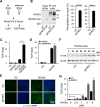
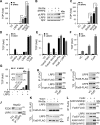
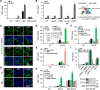
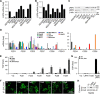
Upon binding to the canonical WNT glycoproteins, Frizzled family receptors (FZDs) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) undergo a series of polymerizations on the cell surface that elicit canonical WNT/β-catenin signaling. The hyperactivation of WNT/β-catenin signaling is the major cause of tumorigenesis, but the mechanism in tumors such as hepatoma remains unclear. Here, we observed that WNT3A manifested the hyperactivity in β-catenin-dependent signaling after binding to FZD's competitive inhibitory molecule secreted Frizzled-related protein 2 (SFRP2). To understand the mechanism of FZDs in the presence of SFRP2, we explored how FZDs can bind and activate the LRP5/6 signalosome independently of WNT glycoproteins. Our findings further revealed that oligomerizations of FZDs and LRP5/6 can integrate the cytoplasmic protein Dishevelled into the LRP5/6 signalosome, resulting in a robust activation of ligand-independent β-catenin signaling. We propose that besides WNT-bridged FZD-WNT-LRP5/6 protein complexes, the homo- and hetero-oligomerizations of WNT receptors may contribute to the formation of the LRP5/6 signalosome on the cell surface. Of note, we identified four highly expressed FZDs in the hepatoma cell line HepG2, all of which significantly promoted ligand-independent LRP5/β-catenin signaling. As FZDs are ectopically expressed in numerous tumors, our findings may provide a new perspective on tumor pathologies. Furthermore, the results in our study suggest that the composition and stoichiometry of FZDs and LRP5/6 within the LRP5/6 signalosome may tune the selection of bound WNT glycoproteins and configure downstream WNT/β-catenin signaling.
Keywords: Frizzled; LRP5/6; SFRP2; Wnt signaling; cancer; cell signaling; hepatoma; receptor endocytosis; signalosome; tumor development.
© 2018 Hua et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
β-Catenin-dependent pathway activation by both promiscuous "canonical" WNT3a-, and specific "noncanonical" WNT4- and WNT5a-FZD receptor combinations with strong differences in LRP5 and LRP6 dependency.Cell Signal. 2014 Feb;26(2):260-7. doi: 10.1016/j.cellsig.2013.11.021. Epub 2013 Nov 21. Cell Signal. 2014. PMID: 24269653
-
Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling.Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a007880. doi: 10.1101/cshperspect.a007880. Cold Spring Harb Perspect Biol. 2012. PMID: 23209147 Free PMC article. Review.
-
WNT-induced association of Frizzled and LRP6 is not sufficient for the initiation of WNT/β-catenin signaling.Nat Commun. 2025 May 24;16(1):4848. doi: 10.1038/s41467-025-60096-7. Nat Commun. 2025. PMID: 40413190 Free PMC article.
-
LRP6 dimerization through its LDLR domain is required for robust canonical Wnt pathway activation.Cell Signal. 2014 May;26(5):1068-74. doi: 10.1016/j.cellsig.2013.12.020. Epub 2014 Jan 8. Cell Signal. 2014. PMID: 24412751
-
Frizzleds and WNT/β-catenin signaling--The black box of ligand-receptor selectivity, complex stoichiometry and activation kinetics.Eur J Pharmacol. 2015 Sep 15;763(Pt B):191-5. doi: 10.1016/j.ejphar.2015.05.031. Epub 2015 May 21. Eur J Pharmacol. 2015. PMID: 26003275 Review.
Cited by
-
Structure and function of the retina of low-density lipoprotein receptor-related protein 5 (Lrp5)-deficient rats.Exp Eye Res. 2022 Apr;217:108977. doi: 10.1016/j.exer.2022.108977. Epub 2022 Feb 6. Exp Eye Res. 2022. PMID: 35139333 Free PMC article.
-
Anti-Proliferative Activity of Nodosin, a Diterpenoid from Isodon serra, via Regulation of Wnt/β-Catenin Signaling Pathways in Human Colon Cancer Cells.Biomol Ther (Seoul). 2020 Sep 1;28(5):465-472. doi: 10.4062/biomolther.2020.003. Biomol Ther (Seoul). 2020. PMID: 32394670 Free PMC article.
-
Structural insights into Frizzled3 through nanobody modulators.Nat Commun. 2024 Aug 22;15(1):7228. doi: 10.1038/s41467-024-51451-1. Nat Commun. 2024. PMID: 39174501 Free PMC article.
-
PI(4,5)P2-stimulated positive feedback drives the recruitment of Dishevelled to Frizzled in Wnt-β-catenin signaling.Sci Signal. 2022 Aug 23;15(748):eabo2820. doi: 10.1126/scisignal.abo2820. Epub 2022 Aug 23. Sci Signal. 2022. PMID: 35998232 Free PMC article.
-
frizzled 5 mutant zebrafish are genetically sensitised to developing microphthalmia and coloboma.Dis Model Mech. 2025 Jun 1;18(6):dmm052284. doi: 10.1242/dmm.052284. Epub 2025 Jun 10. Dis Model Mech. 2025. PMID: 40401611 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

