Structural and kinetic characterization of (S)-1-amino-2-propanol kinase from the aminoacetone utilization microcompartment of Mycobacterium smegmatis
- PMID: 30361441
- PMCID: PMC6314145
- DOI: 10.1074/jbc.RA118.005485
Structural and kinetic characterization of (S)-1-amino-2-propanol kinase from the aminoacetone utilization microcompartment of Mycobacterium smegmatis
Abstract
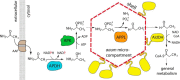
Bacterial microcompartments encapsulate enzymatic pathways that generate small, volatile, aldehyde intermediates. The Rhodococcus and Mycobacterium microcompartment (RMM) operon from Mycobacterium smegmatis encodes four enzymes, including (S)-1-amino-2-propanol dehydrogenase and a likely propionaldehyde dehydrogenase. We show here that a third enzyme (and its nonmicrocompartment-associated paralog) is a moderately specific (S)-1-amino-2-propanol kinase. We determined the structure of apo-aminopropanol kinase at 1.35 Å, revealing that it has structural similarity to hexosamine kinases, choline kinases, and aminoglycoside phosphotransferases. We modeled substrate binding, and tested our model by characterizing key enzyme variants. Bioinformatics analysis established that this enzyme is widespread in Actinobacteria, Proteobacteria, and Firmicutes, and is very commonly associated with a candidate phospholyase. In Rhizobia, aminopropanol kinase is generally associated with aromatic degradation pathways. In the RMM (and the parallel pathway that includes the second paralog), aminopropanol kinase likely degrades aminoacetone through a propanolamine-phosphate phospho-lyase-dependent pathway. These enzymatic activities were originally described in Pseudomonas, but the proteins responsible have not been previously identified. Bacterial microcompartments typically co-encapsulate enzymes which can regenerate required co-factors, but the RMM enzymes require four biochemically distinct co-factors with no overlap. This suggests that either the RMM shell can uniquely transport multiple co-factors in stoichiometric quantities, or that all enzymes except the phospho-lyase reside outside of the shell. In summary, aminopropanol kinase is a novel enzyme found in diverse bacteria and multiple metabolic pathways; its presence in the RMM implies that this microcompartment degrades aminoacetone, using a pathway that appears to violate some established precepts as to how microcompartments function.
Keywords: aminoacetone utilization microcompartment; aminopropanol kinase; biodegradation; bioinformatics; enzyme kinetics; microbiology; protein structure.
© 2018 Mallette and Kimber.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

