Rsp5 Ubiquitin ligase-mediated quality control system clears membrane proteins mistargeted to the vacuole membrane
- PMID: 30361468
- PMCID: PMC6314561
- DOI: 10.1083/jcb.201806094
Rsp5 Ubiquitin ligase-mediated quality control system clears membrane proteins mistargeted to the vacuole membrane
Abstract
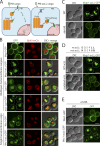
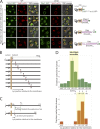
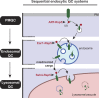
Maintenance of organelle identity is profoundly dependent on the coordination between correct targeting of proteins and removal of mistargeted and damaged proteins. This task is mediated by organelle-specific protein quality control (QC) systems. In yeast, the endocytosis and QC of most plasma membrane (PM) proteins requires the Rsp5 ubiquitin ligase and ART adaptor network. We show that intracellular adaptors of Rsp5, Ear1, and Ssh4 mediate recognition and vacuolar degradation of PM proteins that escape or bypass PM QC systems. This second tier of surveillance helps to maintain cell integrity upon heat stress and protects from proteotoxicity. To understand the mechanism of the recognition of aberrant PM cargos by Ssh4-Rsp5, we mistarget multiple PM proteins de novo to the vacuolar membrane. We found that Ssh4-Rsp5 can target and ubiquitinate multiple lysines within a restricted distance from the membrane, providing a fail-safe mechanism for a diverse cargo repertoire. The mistargeting or misfolding of PM proteins likely exposes these lysines or shifts them into the "ubiquitination zone" accessible to the Ssh4-Rsp5 complex.
© 2018 Sardana et al.
Figures






Similar articles
-
Downregulation of the broad-specificity amino acid permease Agp1 mediated by the ubiquitin ligase Rsp5 and the arrestin-like protein Bul1 in yeast.Biosci Biotechnol Biochem. 2021 Apr 24;85(5):1266-1274. doi: 10.1093/bbb/zbab028. Biosci Biotechnol Biochem. 2021. PMID: 33620458
-
The ART-Rsp5 ubiquitin ligase network comprises a plasma membrane quality control system that protects yeast cells from proteotoxic stress.Elife. 2013 Apr 16;2:e00459. doi: 10.7554/eLife.00459. Elife. 2013. PMID: 23599894 Free PMC article.
-
GGA2- and ubiquitin-dependent trafficking of Arn1, the ferrichrome transporter of Saccharomyces cerevisiae.Mol Biol Cell. 2007 May;18(5):1790-802. doi: 10.1091/mbc.e06-09-0861. Epub 2007 Mar 7. Mol Biol Cell. 2007. PMID: 17344478 Free PMC article.
-
The ubiquitin code of yeast permease trafficking.Trends Cell Biol. 2010 Apr;20(4):196-204. doi: 10.1016/j.tcb.2010.01.004. Trends Cell Biol. 2010. PMID: 20138522 Review.
-
Role of Rsp5 ubiquitin ligase in biogenesis of rRNA, mRNA and tRNA in yeast.RNA Biol. 2015;12(12):1265-74. doi: 10.1080/15476286.2015.1094604. RNA Biol. 2015. PMID: 26403176 Free PMC article. Review.
Cited by
-
A bipartite sorting signal ensures specificity of retromer complex in membrane protein recycling.J Cell Biol. 2019 Sep 2;218(9):2876-2886. doi: 10.1083/jcb.201901019. Epub 2019 Jul 23. J Cell Biol. 2019. PMID: 31337624 Free PMC article.
-
Impact of Membrane Lipids on UapA and AzgA Transporter Subcellular Localization and Activity in Aspergillus nidulans.J Fungi (Basel). 2021 Jun 28;7(7):514. doi: 10.3390/jof7070514. J Fungi (Basel). 2021. PMID: 34203131 Free PMC article.
-
ESCRT, not intralumenal fragments, sorts ubiquitinated vacuole membrane proteins for degradation.J Cell Biol. 2021 Aug 2;220(8):e202012104. doi: 10.1083/jcb.202012104. Epub 2021 May 28. J Cell Biol. 2021. PMID: 34047770 Free PMC article.
-
Lysosomal membrane transporter purification and reconstitution for functional studies.Mol Biol Cell. 2024 Mar 1;35(3):ar28. doi: 10.1091/mbc.E23-06-0259. Epub 2023 Dec 20. Mol Biol Cell. 2024. PMID: 38117592 Free PMC article.
-
Retrograde trafficking and quality control of yeast synaptobrevin, Snc1, are conferred by its transmembrane domain.Mol Biol Cell. 2019 Jul 1;30(14):1729-1742. doi: 10.1091/mbc.E19-02-0117. Epub 2019 May 8. Mol Biol Cell. 2019. PMID: 31067149 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

