Structural dynamics of the E6AP/UBE3A-E6-p53 enzyme-substrate complex
- PMID: 30361475
- PMCID: PMC6202321
- DOI: 10.1038/s41467-018-06953-0
Structural dynamics of the E6AP/UBE3A-E6-p53 enzyme-substrate complex
Abstract
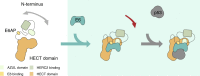
Deregulation of the ubiquitin ligase E6AP is causally linked to the development of human disease, including cervical cancer. In complex with the E6 oncoprotein of human papillomaviruses, E6AP targets the tumor suppressor p53 for degradation, thereby contributing to carcinogenesis. Moreover, E6 acts as a potent activator of E6AP by a yet unknown mechanism. However, structural information explaining how the E6AP-E6-p53 enzyme-substrate complex is assembled, and how E6 stimulates E6AP, is largely missing. Here, we develop and apply different crosslinking mass spectrometry-based approaches to study the E6AP-E6-p53 interplay. We show that binding of E6 induces conformational rearrangements in E6AP, thereby positioning E6 and p53 in the immediate vicinity of the catalytic center of E6AP. Our data provide structural and functional insights into the dynamics of the full-length E6AP-E6-p53 enzyme-substrate complex, demonstrating how E6 can stimulate the ubiquitin ligase activity of E6AP while facilitating ubiquitin transfer from E6AP onto p53.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- SFB 969/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- BB/N015274/1/Biotechnology and Biological Sciences Research Council (BBSRC)/International
- Wellcome Trust/United Kingdom
- STE 2517/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- 104194/Z/14/Z/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

