A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects
- PMID: 30361486
- PMCID: PMC6202397
- DOI: 10.1038/s41467-018-06504-7
A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects
Abstract
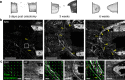
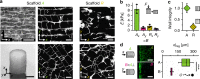
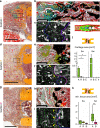
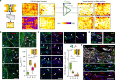
Biomaterials developed to treat bone defects have classically focused on bone healing via direct, intramembranous ossification. In contrast, most bones in our body develop from a cartilage template via a second pathway called endochondral ossification. The unsolved clinical challenge to regenerate large bone defects has brought endochondral ossification into discussion as an alternative approach for bone healing. However, a biomaterial strategy for the regeneration of large bone defects via endochondral ossification is missing. Here we report on a biomaterial with a channel-like pore architecture to control cell recruitment and tissue patterning in the early phase of healing. In consequence of extracellular matrix alignment, CD146+ progenitor cell accumulation and restrained vascularization, a highly organized endochondral ossification process is induced in rats. Our findings demonstrate that a pure biomaterial approach has the potential to recapitulate a developmental bone growth process for bone healing. This might motivate future strategies for biomaterial-based tissue regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Recapitulating endochondral ossification: a promising route to in vivo bone regeneration.J Tissue Eng Regen Med. 2015 Aug;9(8):889-902. doi: 10.1002/term.1918. Epub 2014 Jun 11. J Tissue Eng Regen Med. 2015. PMID: 24916192 Review.
-
Incorporation of metal-doped silicate microparticles into collagen scaffolds combines chemical and architectural cues for endochondral bone healing.Acta Biomater. 2025 Jan 15;192:260-278. doi: 10.1016/j.actbio.2024.12.029. Epub 2024 Dec 12. Acta Biomater. 2025. PMID: 39674241
-
Fractionated human adipose tissue as a native biomaterial for the generation of a bone organ by endochondral ossification.Acta Biomater. 2018 Sep 1;77:142-154. doi: 10.1016/j.actbio.2018.07.004. Epub 2018 Jul 4. Acta Biomater. 2018. PMID: 30126590
-
Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization.Acta Biomater. 2018 Dec;82:1-11. doi: 10.1016/j.actbio.2018.10.016. Epub 2018 Oct 13. Acta Biomater. 2018. PMID: 30321630 Free PMC article.
-
Endochondral ossification for enhancing bone regeneration: converging native extracellular matrix biomaterials and developmental engineering in vivo.Tissue Eng Part B Rev. 2015 Jun;21(3):247-66. doi: 10.1089/ten.TEB.2014.0419. Epub 2014 Dec 4. Tissue Eng Part B Rev. 2015. PMID: 25336144 Free PMC article. Review.
Cited by
-
Role of NEL‑like molecule‑1 in osteogenesis/chondrogenesis (Review).Int J Mol Med. 2025 Jan;55(1):5. doi: 10.3892/ijmm.2024.5446. Epub 2024 Oct 25. Int J Mol Med. 2025. PMID: 39450541 Free PMC article. Review.
-
Close-to-native bone repair via tissue-engineered endochondral ossification approaches.iScience. 2022 Oct 14;25(11):105370. doi: 10.1016/j.isci.2022.105370. eCollection 2022 Nov 18. iScience. 2022. PMID: 36339269 Free PMC article. Review.
-
Advanced therapies in orthopaedics.EFORT Open Rev. 2024 Sep 2;9(9):837-844. doi: 10.1530/EOR-24-0084. EFORT Open Rev. 2024. PMID: 39222330 Free PMC article. Review.
-
Diffractive small angle X-ray scattering imaging for anisotropic structures.Nat Commun. 2019 Nov 12;10(1):5130. doi: 10.1038/s41467-019-12635-2. Nat Commun. 2019. PMID: 31719528 Free PMC article.
-
Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration.Int J Oral Sci. 2020 Feb 6;12(1):6. doi: 10.1038/s41368-020-0073-y. Int J Oral Sci. 2020. PMID: 32024822 Free PMC article. Review.
References
-
- Emans P. J. C., M. M. J., van Rhijn, L. W., Welting, T. J. M. in Tissue Regeneration—FromBasic Biology to Clinical Application (eds Davies J.) (InTech, 2012).
-
- Stewart, S., Bryant, S. J., Ahn, J., Hankenson, K. D. in Translational Regenerative Medicine (eds Atala, A. & Allickson, J.) (Elsevier Academic Press, 2014).
Publication types
MeSH terms
Substances
Grants and funding
- 13N12154/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)/International
- 13N12152/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)/International
- PE 1802/1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

