A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease
- PMID: 30361506
- PMCID: PMC6202333
- DOI: 10.1038/s41467-018-06964-x
A homozygous loss-of-function mutation leading to CYBC1 deficiency causes chronic granulomatous disease
Abstract
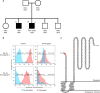
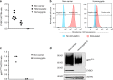
Mutations in genes encoding subunits of the phagocyte NADPH oxidase complex are recognized to cause chronic granulomatous disease (CGD), a severe primary immunodeficiency. Here we describe how deficiency of CYBC1, a previously uncharacterized protein in humans (C17orf62), leads to reduced expression of NADPH oxidase's main subunit (gp91phox) and results in CGD. Analyzing two brothers diagnosed with CGD we identify a homozygous loss-of-function mutation, p.Tyr2Ter, in CYBC1. Imputation of p.Tyr2Ter into 155K chip-genotyped Icelanders reveals six additional homozygotes, all with signs of CGD, manifesting as colitis, rare infections, or a severely impaired PMA-induced neutrophil oxidative burst. Homozygosity for p.Tyr2Ter consequently associates with inflammatory bowel disease (IBD) in Iceland (P = 8.3 × 10-8; OR = 67.6), as well as reduced height (P = 3.3 × 10-4; -8.5 cm). Overall, we find that CYBC1 deficiency results in CGD characterized by colitis and a distinct profile of infections indicative of macrophage dysfunction.
Conflict of interest statement
The authors affiliated with deCODE genetics/Amgen declare a conflict of interest as employees. The remaining authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

