Angiotensin 1-7 modulates molecular and cellular processes central to the pathogenesis of prostate cancer
- PMID: 30361641
- PMCID: PMC6202343
- DOI: 10.1038/s41598-018-34049-8
Angiotensin 1-7 modulates molecular and cellular processes central to the pathogenesis of prostate cancer
Abstract
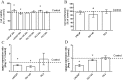
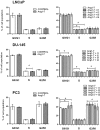
Angiotensin 1-7 (Ang1-7) is an endogenous bioactive component of the renin-angiotensin system (RAS). In addition to its cardiovascular properties, its anti-proliferative and anti-angiogenic traits are believed to play important roles in carcinogenesis. The present study examines the influence of Ang1-7 on processes associated with development and progression of prostate cancer cells. Our findings indicate that while Ang1-7 (1 nM; 48 h) can effectively reduce cell proliferation in DU-145, it can induce a significant decrease in the expression of MKI67 in LNCaP. In both cell lines we also observed a reduction in colony size in soft agar assay. A various changes in gene expression were noted after exposure to Ang1-7: those of anti- and pro-apoptotic agents and the NF-kB family of transcription factors, as well as mesenchymal cell markers and vascular endothelial growth factor A (VEGFA). In addition, Ang1-7 was found to modulate cell adhesion and matrix metallopeptidase (MMP) activity. Changes were also observed in the levels of angiotensin receptors and sex steroid hormone receptors. Ang1-7 reduced the levels of estrogen receptor alpha gene (ESR1) and increased the expression of estrogen receptor beta gene (ESR2) in all prostate cancer cells; it also up-regulated androgen receptor (AR) expression in androgen-sensitive cells but contradictory effect was observed in androgen- irresponsive cell lines. In summary, the results confirm the existence of complex network between the various elements of the local RAS and the molecular and cellular mechanisms of prostate cancerogenesis. The response of cancer cells to Ang1-7 appears to vary dependently on the dose and time of incubation as well as the aggressiveness and the hormonal status of cells.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Domińska K, Lachowicz-Ochedalska A. The involvement of the renin-angiotensin system (RAS) in cancerogenesis. Postepy Biochem. 2008;54:294–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

