Roles of Cytochrome P450 in Metabolism of Ethanol and Carcinogens
- PMID: 30362088
- PMCID: PMC6371814
- DOI: 10.1007/978-3-319-98788-0_2
Roles of Cytochrome P450 in Metabolism of Ethanol and Carcinogens
Abstract
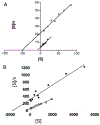
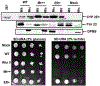
Cytochrome P450 (P450) enzymes are involved in the metabolism of carcinogens, as well as drugs, steroids, vitamins, and other classes of chemicals. P450s also oxidize ethanol, in particular P450 2E1. P450 2E1 oxidizes ethanol to acetaldehyde and then to acetic acid, roles also played by alcohol and aldehyde dehydrogenases. The role of P450 2E1 in cancer is complex in that P450 2E1 is also induced by ethanol, P450 2E1 is involved in the bioactivation and detoxication of a number of chemical carcinogens, and ethanol is an inhibitor of P450 2E1. Contrary to some literature, P450 2E1 expression and induction itself does not cause global oxidative stress in vivo, as demonstrated in studies using isoniazid treatment and gene deletion studies with rats and mice. However, a major fraction of P450 2E1 is localized in liver mitochondria instead of the endoplasmic reticulum, and studies with site-directed rat P450 2E1 mutants and natural human P450 2E1 N-terminal variants have shown that P450 2E1 localized in mitochondria is catalytically active and more proficient in producing reactive oxygen species and damage. The role of the mitochondrial oxidative stress in ethanol toxicity is still under investigation, as is the mechanism of altered electron transport to P450s that localize inside mitochondria instead of their typical endoplasmic reticulum environment.
Keywords: Chemical carcinogens; Cytochrome P450 2E1; Endoplasmic reticulum, and cytochrome P450; Enzyme kinetics; Ethanol; Mitochondria, and cytochrome P450; Mitochondrial toxicity; Oxidation by cytochrome P450, in ethanol oxidation; P450 2E1; Reactive oxygen species.
Figures







References
-
- Anandatheerthavarada HK, Addya S, Mullick J, Avadhani NG (1998) Interaction of adrenodoxin with P4501A1 and its truncated form P450MT2 through different domains: differential modulation of enzyme activities. Biochemistry 37:1150–1160 - PubMed
-
- Backer JM, Weinstein IB (1980) Mitochondrial DNA is a major cellular target for a dihydrodiol- epoxide derivative of benzo[a]pyrene. Science 209:297–299 - PubMed
-
- Bajpai P, Sangar MC, Singh S, Tang W, Bansal S, Chowdhury G, Cheng Q, Fang JK, Martin MV, Guengerich FP, Avadhani NG (2013) Metabolism of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease. J Biol Chem 288:4436–4451 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

