Day-to-day fasting self-monitored blood glucose variability is associated with risk of hypoglycaemia in insulin-treated patients with type 1 and type 2 diabetes: A post hoc analysis of the SWITCH Trials
- PMID: 30362250
- PMCID: PMC6587774
- DOI: 10.1111/dom.13565
Day-to-day fasting self-monitored blood glucose variability is associated with risk of hypoglycaemia in insulin-treated patients with type 1 and type 2 diabetes: A post hoc analysis of the SWITCH Trials
Abstract
Aims: To investigate the association between day-to-day fasting self-monitored blood glucose (SMBG) variability and risk of hypoglycaemia in type 1 (T1D) and type 2 diabetes (T2D), and to compare day-to-day fasting SMBG variability between treatments with insulin degludec (degludec) and insulin glargine 100 units/mL (glargine U100).
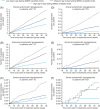
Materials and methods: Data were retrieved from two double-blind, randomized, treat-to-target, two-period (32 weeks each) crossover trials of degludec vs glargine U100 in T1D (SWITCH 1, n = 501) and T2D (SWITCH 2, n = 720). Available fasting SMBGs were used to determine the standard deviation (SD) of day-to-day fasting SMBG variability for each patient and the treatment combination. The association between day-to-day fasting SMBG variability and overall symptomatic, nocturnal symptomatic and severe hypoglycaemia was analysed for the pooled population using linear regression, with fasting SMBG variability included as a three-level factor defined by population tertiles. Finally, day-to-day fasting SMBG variability was compared between treatments.
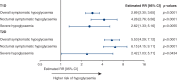
Results: Linear regression showed that day-to-day fasting SMBG variability was significantly associated with overall symptomatic, nocturnal symptomatic and severe hypoglycaemia risk in T1D and T2D (P < 0.05). Day-to-day fasting SMBG variability was significantly associated (P < 0.01) with all categories of hypoglycaemia risk, with the exception of severe hypoglycaemia in T2D when analysed within tertiles. Degludec was associated with 4% lower day-to-day fasting SMBG variability than glargine U100 in T1D (P = 0.0082) and with 10% lower day-to-day fasting SMBG variability in T2D (P < 0.0001).
Conclusions: Higher day-to-day fasting SMBG variability is associated with an increased risk of overall symptomatic, nocturnal symptomatic and severe hypoglycaemia. Degludec has significantly lower day-to-day fasting SMBG variability vs glargine U100.
Keywords: basal insulin; hypoglycaemia; insulin therapy; type 1 diabetes; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Author contributions
All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship. Specifically, all authors made substantial contributions to the interpretation of data for the manuscript, drafted and critically revised the manuscript, provided final approval of the version to be published and agreed to be accountable for all aspects of the manuscript. All authors had access to the final results and vouch for the fidelity of the trial to the protocol. All authors are responsible for the integrity of the work as a whole.
Figures
References
-
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977‐986. - PubMed
-
- Cryer PE. Glycemic goals in diabetes: trade‐off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188‐2195. - PubMed
-
- Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711‐722. - PubMed

