Cancer cachexia impairs neural respiratory drive in hypoxia but not hypercapnia
- PMID: 30362273
- PMCID: PMC6438337
- DOI: 10.1002/jcsm.12348
Cancer cachexia impairs neural respiratory drive in hypoxia but not hypercapnia
Abstract
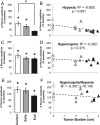
Background: Cancer cachexia is an insidious process characterized by muscle atrophy with associated motor deficits, including diaphragm weakness and respiratory insufficiency. Although neuropathology contributes to muscle wasting and motor deficits in many clinical disorders, neural involvement in cachexia-linked respiratory insufficiency has not been explored.
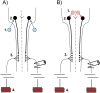
Methods: We first used whole-body plethysmography to assess ventilatory responses to hypoxic and hypercapnic chemoreflex activation in mice inoculated with the C26 colon adenocarcinoma cell line. Mice were exposed to a sequence of inspired gas mixtures consisting of (i) air, (ii) hypoxia (11% O2 ) with normocapnia, (iii) hypercapnia (7% CO2 ) with normoxia, and (iv) combined hypercapnia with hypoxia (i.e. maximal chemoreflex response). We also tested the respiratory neural network directly by recording inspiratory burst output from ligated phrenic nerves, thereby bypassing influences from changes in diaphragm muscle strength, respiratory mechanics, or compensation through recruitment of accessory motor pools.
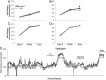
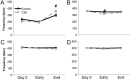
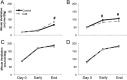
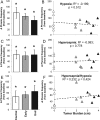
Results: Cachectic mice demonstrated a significant attenuation of the hypoxic tidal volume (0.26mL±0.01mL vs 0.30mL±0.01mL; p<0.05), breathing frequency (317±10bpm vs 344±6bpm; p<0.05) and phrenic nerve (29.5±2.6% vs 78.8±11.8%; p<0.05) responses. On the other hand, the much larger hypercapnic tidal volume (0.46±0.01mL vs 0.46±0.01mL; p>0.05), breathing frequency (392±5bpm vs 408±5bpm; p>0.05) and phrenic nerve (93.1±8.8% vs 111.1±13.2%; p>0.05) responses were not affected. Further, the concurrent hypercapnia/hypoxia tidal volume (0.45±0.01mL vs 0.45±0.01mL; p>0.05), breathing frequency (395±7bpm vs 400±3bpm; p>0.05), and phrenic nerve (106.8±7.1% vs 147.5±38.8%; p>0.05) responses were not different between C26 cachectic and control mice.
Conclusions: Breathing deficits associated with cancer cachexia are specific to the hypoxic ventilatory response and, thus, reflect disruptions in the hypoxic chemoafferent neural network. Diagnostic techniques that detect decompensation and therapeutic approaches that support the failing hypoxic respiratory response may benefit patients at risk for cancer cachectic-associated respiratory failure.
Keywords: Breathing; Cancer; Chemoreflex and hypoxia.
© 2018 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Argilés JM, Busquets S, Toledo M, López‐Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 2009;3:263–268. - PubMed
-
- Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010;142:531–543. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

