Synthesis and biological evaluation of 3-arylcoumarin derivatives as potential anti-diabetic agents
- PMID: 30362362
- PMCID: PMC6211316
- DOI: 10.1080/14756366.2018.1518958
Synthesis and biological evaluation of 3-arylcoumarin derivatives as potential anti-diabetic agents
Abstract
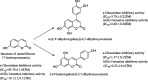
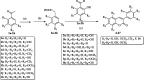
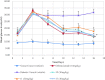
A variety of substituted 3-arylcoumarin derivatives were synthesised through microwave radiation heating. The method has characteristics of environmental friendliness, economy, simple separation, and purification process, less by-products and high reaction yield. Those 3-arylcoumarin derivatives were screened for antioxidant, α-glucosidase inhibitory and advanced glycation end-products (AGEs) formation inhibitory. Most compounds exhibited significant antioxidant and AGEs formation inhibitory activities. Anti-diabetic activity studies showed that compounds 11 and 17 were equipotent to the standard drug glibenclamide in vivo. According to the experimental results, the target compound 35 can be used as a lead compound for the development of new anti-diabetic drugs. The whole experiment showed that anti-diabetic activity is prevalent in 3-arylcoumarins, which added a new natural skeleton to the development of anti-diabetic active drugs.
Keywords: 3-Arylcoumarin; AGEs inhibitor; antidiabetic; α-glucosidase inhibitor.
Figures
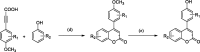

Similar articles
-
Synthesis & α-glucosidase inhibitory & glucose consumption-promoting activities of flavonoid-coumarin hybrids.Future Med Chem. 2018 May 1;10(9):1055-1066. doi: 10.4155/fmc-2017-0293. Epub 2018 Apr 20. Future Med Chem. 2018. PMID: 29676183
-
Design, synthesis and evaluation of phenylfuroxan nitric oxide-donor phenols as potential anti-diabetic agents.Bioorg Chem. 2019 Aug;89:103000. doi: 10.1016/j.bioorg.2019.103000. Epub 2019 May 20. Bioorg Chem. 2019. PMID: 31132604
-
Synthesis, antidiabetic activity and molecular docking study of rhodanine-substitued spirooxindole pyrrolidine derivatives as novel α-amylase inhibitors.Bioorg Chem. 2021 Jan;106:104507. doi: 10.1016/j.bioorg.2020.104507. Epub 2020 Nov 26. Bioorg Chem. 2021. PMID: 33288322
-
Coumarin-based Scaffold as α-glucosidase Inhibitory Activity: Implication for the Development of Potent Antidiabetic Agents.Mini Rev Med Chem. 2020;20(2):134-151. doi: 10.2174/1389557519666190925162536. Mini Rev Med Chem. 2020. PMID: 31553294 Review.
-
Screening of antidiabetic and antioxidant activities of medicinal plants.J Integr Med. 2015 Sep;13(5):297-305. doi: 10.1016/S2095-4964(15)60193-5. J Integr Med. 2015. PMID: 26343100 Review.
Cited by
-
Research progress of coumarins and their derivatives in the treatment of diabetes.J Enzyme Inhib Med Chem. 2022 Dec;37(1):616-628. doi: 10.1080/14756366.2021.2024526. J Enzyme Inhib Med Chem. 2022. PMID: 35067136 Free PMC article. Review.
-
3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry: The Landmarks of the Past Decade.Molecules. 2021 Nov 8;26(21):6755. doi: 10.3390/molecules26216755. Molecules. 2021. PMID: 34771164 Free PMC article. Review.
-
Exploring the molecular mechanism of Si-Miao-Yong-An Decoction in treating diabetic foot using network pharmacological analysis and molecular docking technique.Medicine (Baltimore). 2025 May 30;104(22):e42629. doi: 10.1097/MD.0000000000042629. Medicine (Baltimore). 2025. PMID: 40441214 Free PMC article.
-
Discovery of Novel Coumarin Analogs against the α-Glucosidase Protein Target of Diabetes Mellitus: Pharmacophore-Based QSAR, Docking, and Molecular Dynamics Simulation Studies.ACS Omega. 2020 Dec 10;5(50):32234-32249. doi: 10.1021/acsomega.0c03871. eCollection 2020 Dec 22. ACS Omega. 2020. PMID: 33376861 Free PMC article.
-
Natural source, bioactivity and synthesis of 3-Arylcoumarin derivatives.J Enzyme Inhib Med Chem. 2022 Dec;37(1):1023-1042. doi: 10.1080/14756366.2022.2058499. J Enzyme Inhib Med Chem. 2022. PMID: 35438580 Free PMC article. Review.
References
-
- Kabeya LM, de Marchi AA, Kanashiro A, et al. . Inhibition of horseradish peroxidase catalytic activity by new 3-phenylcoumarin derivatives: synthesis and structure-activity relationships. Bioorg Med Chem. 2007;15:1516–24. - PubMed
-
- Wang B, Li N, Liu T, et al. . Synthesis and biological evaluation of novel neoflavonoid derivatives as potential antidiabetic agents. RSC Adv. 2017;7:34448–60.
-
- Keresztury G, Holly S, Komlósi V, et al. . Analysis of the vibrational spectra of new OH-containing E-4-arylmethylene-3-isochromanones and 3-arylcoumarins. J Biochem Biophys Methods. 2006;69:163–77. - PubMed
-
- Billard C, Menasria F, Quiney C, et al. . 4-Arylcoumarin analogues of combretastatins stimulate apoptosis of leukemic cells from chronic lymphocytic leukemia patients. Exp Hematol. 2008;36:1625–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
