Refining the structure-activity relationships of 2-phenylcyclopropane carboxylic acids as inhibitors of O-acetylserine sulfhydrylase isoforms
- PMID: 30362368
- PMCID: PMC6217552
- DOI: 10.1080/14756366.2018.1518959
Refining the structure-activity relationships of 2-phenylcyclopropane carboxylic acids as inhibitors of O-acetylserine sulfhydrylase isoforms
Abstract
The lack of efficacy of current antibacterials to treat multidrug resistant bacteria poses a life-threatening alarm. In order to develop enhancers of the antibacterial activity, we carried out a medicinal chemistry campaign aiming to develop inhibitors of enzymes that synthesise cysteine and belong to the reductive sulphur assimilation pathway, absent in mammals. Previous studies have provided a novel series of inhibitors for O-acetylsulfhydrylase - a key enzyme involved in cysteine biosynthesis. Despite displaying nanomolar affinity, the most active representative of the series was not able to interfere with bacterial growth, likely due to poor permeability. Therefore, we rationally modified the structure of the hit compound with the aim of promoting their passage through the outer cell membrane porins. The new series was evaluated on the recombinant enzyme from Salmonella enterica serovar Typhimurium, with several compounds able to keep nanomolar binding affinity despite the extent of chemical manipulation.
Keywords: Antibacterials; Gram-negatives; O-acetylserine sulfhydrylase; cysteine; permeability.
Figures



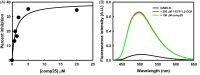

References
-
- Campanini B, Pieroni M, Raboni S, et al. . Inhibitors of the sulfur assimilation pathway in bacterial pathogens as enhancers of antibiotic therapy. Curr Med Chem 2015;22:187–213. - PubMed
-
- Mozzarelli A, Bettati S, Campanini B, et al. . The multifaceted pyridoxal 5’-phosphate-dependent O-acetylserine sulfhydrylase. Biochim Biophys Acta 2011;1814:1497–510. - PubMed
-
- Spyrakis F, Felici P, Bayden AS, et al. . Fine tuning of the active site modulates specificity in the interaction of O-acetylserine sulfhydrylase isozymes with serine acetyltransferase. Biochimica et Biophysica Acta (BBA) − Proteins and Proteomics 2013;1834:169–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
