Functional trade-offs and environmental variation shaped ancient trajectories in the evolution of dim-light vision
- PMID: 30362942
- PMCID: PMC6203435
- DOI: 10.7554/eLife.35957
Functional trade-offs and environmental variation shaped ancient trajectories in the evolution of dim-light vision
Abstract
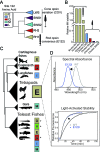
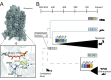
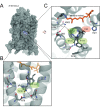
Trade-offs between protein stability and activity can restrict access to evolutionary trajectories, but widespread epistasis may facilitate indirect routes to adaptation. This may be enhanced by natural environmental variation, but in multicellular organisms this process is poorly understood. We investigated a paradoxical trajectory taken during the evolution of tetrapod dim-light vision, where in the rod visual pigment rhodopsin, E122 was fixed 350 million years ago, a residue associated with increased active-state (MII) stability but greatly diminished rod photosensitivity. Here, we demonstrate that high MII stability could have likely evolved without E122, but instead, selection appears to have entrenched E122 in tetrapods via epistatic interactions with nearby coevolving sites. In fishes by contrast, selection may have exploited these epistatic effects to explore alternative trajectories, but via indirect routes with low MII stability. Our results suggest that within tetrapods, E122 and high MII stability cannot be sacrificed-not even for improvements to rod photosensitivity.
Keywords: biochemistry; chemical biology; evolutionary biology; intramolecular epistasis; none; protein evolution; rhodopsin.
© 2018, Castiglione et al.
Conflict of interest statement
GC, BC No competing interests declared
Figures







References
-
- Amemiya CT, Alföldi J, Lee AP, Fan S, Philippe H, MacCallum I, Braasch I, Manousaki T, Schneider I, Rohner N, Organ C, Chalopin D, Smith JJ, Robinson M, Dorrington RA, Gerdol M, Aken B, Biscotti MA, Barucca M, Baurain D, Berlin AM, Blatch GL, Buonocore F, Burmester T, Campbell MS, Canapa A, Cannon JP, Christoffels A, De Moro G, Edkins AL, Fan L, Fausto AM, Feiner N, Forconi M, Gamieldien J, Gnerre S, Gnirke A, Goldstone JV, Haerty W, Hahn ME, Hesse U, Hoffmann S, Johnson J, Karchner SI, Kuraku S, Lara M, Levin JZ, Litman GW, Mauceli E, Miyake T, Mueller MG, Nelson DR, Nitsche A, Olmo E, Ota T, Pallavicini A, Panji S, Picone B, Ponting CP, Prohaska SJ, Przybylski D, Saha NR, Ravi V, Ribeiro FJ, Sauka-Spengler T, Scapigliati G, Searle SMJ, Sharpe T, Simakov O, Stadler PF, Stegeman JJ, Sumiyama K, Tabbaa D, Tafer H, Turner-Maier J, van Heusden P, White S, Williams L, Yandell M, Brinkmann H, Volff J-N, Tabin CJ, Shubin N, Schartl M, Jaffe DB, Postlethwait JH, Venkatesh B, Di Palma F, Lander ES, Meyer A, Lindblad-Toh K. The african coelacanth genome provides insights into tetrapod evolution. Nature. 2013;496:311–316. doi: 10.1038/nature12027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

