The 3-Phosphoinositide-Dependent Protein Kinase 1 Inhibits Rod Photoreceptor Development
- PMID: 30364083
- PMCID: PMC6191476
- DOI: 10.3389/fcell.2018.00134
The 3-Phosphoinositide-Dependent Protein Kinase 1 Inhibits Rod Photoreceptor Development
Abstract
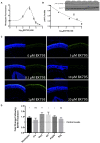
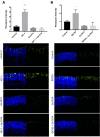
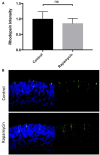
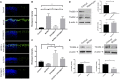
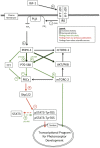
The transition of rod precursor cells to post-mitotic rod photoreceptors can be promoted by extrinsic factors such as insulin-like growth factor 1 (IGF-1), which regulates phosphatidylinositide concentration, and consequently the 3-phosphoinositide-dependent protein kinase-1 (PDPK-1). PDPK-1 is a 63 kDa cytoplasmic kinase that controls cell proliferation and differentiation. In the mouse retina, PDPK-1 and its phosphorylated derivative p-PDPK-1 (Ser241), showed peak expression during the first postnatal (PN) day with a substantial decline by PN7 and in the adult retina. Though initially widely distributed among cell types, PDPK-1 expression decreased first in the inner retina and later in the outer retina. When PDPK-1 is inhibited in neonatal retinal explants by BX795, there is a robust increase in rod photoreceptor numbers. The increase in rods depended on the activity of PKC, as BX795 had no effect when PKC is inhibited. Inhibition of PDPK-1-dependent kinases, such as P70-S6K, but not others, such as mTORC-1, stimulated rod development. The P70-S6K-dependent increase in rods appears to be correlated with phosphorylation of Thr252 and not at Thr389, a substrate of mTORC-1. This pathway is also inactive while PKC activity is inhibited. We also found that inhibition of the kinase mTORC-2, also stimulated by insulin activity, similarly increased rod formation, and this effect appears to be independent of PKC activity. This may represent a novel intracellular signaling pathway that also stimulates photoreceptor development. Consistent with previous studies, stimulation of STAT3 activity is sufficient to prevent any PDPK-1, P70-S6K, or mTORC2-dependent increase in rods. Together the data indicate that PDPK-1 and other intrinsic kinases downstream of IGF-1 are key regulators of rod photoreceptor formation.
Keywords: IGF-1; PDPK-1; PKC; STAT3; development; mTORC2; photoreceptor; retina.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

