Cellular and Molecular Immune Response to Chikungunya Virus Infection
- PMID: 30364124
- PMCID: PMC6191487
- DOI: 10.3389/fcimb.2018.00345
Cellular and Molecular Immune Response to Chikungunya Virus Infection
Abstract
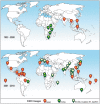
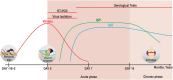
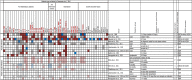
Chikungunya virus (CHIKV) is a re-emergent arthropod-borne virus (arbovirus) that causes a disease characterized primarily by fever, rash and severe persistent polyarthralgia. In the last decade, CHIKV has become a serious public health problem causing several outbreaks around the world. Despite the fact that CHIKV has been around since 1952, our knowledge about immunopathology, innate and adaptive immune response involved in this infectious disease is incomplete. In this review, we provide an updated summary of the current knowledge about immune response to CHIKV and about soluble immunological markers associated with the morbidity, prognosis and chronicity of this arbovirus disease. In addition, we discuss the progress in the research of new vaccines for preventing CHIKV infection and the use of monoclonal antibodies as a promising therapeutic strategy.
Keywords: Chikungunya virus; adaptative immunity; immune response; immunological markers; immunovirology; innate immunity; vaccines.
Figures

References
-
- Ahola T., Merits A. (2016). Functions of Chikungunya virus nonstructural proteins, in Chikungunya Virus: Advances in Biology, Pathogenesis, and Treatment, ed Okeoma C. M. (Cham: Springer International Publishing; ), 75–98.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

