Sulfur Transport and Metabolism in Legume Root Nodules
- PMID: 30364181
- PMCID: PMC6192434
- DOI: 10.3389/fpls.2018.01434
Sulfur Transport and Metabolism in Legume Root Nodules
Abstract
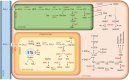
Sulfur is an essential nutrient in plants as a constituent element of some amino acids, metal cofactors, coenzymes, and secondary metabolites. Not surprisingly, sulfur deficiency decreases plant growth, photosynthesis, and seed yield in both legumes and non-legumes. In nodulated legumes, sulfur supply is positively linked to symbiotic nitrogen fixation (SNF) and sulfur starvation causes three additional major effects: decrease of nodulation, inhibition of SNF, and slowing down of nodule metabolism. These effects are due, at least in part, to the impairment of nitrogenase biosynthesis and activity, the accumulation of nitrogen-rich amino acids, and the decline in leghemoglobin, ferredoxin, ATP, and glucose in nodules. During the last decade, some major advances have been made about the uptake and metabolism of sulfur in nodules. These include the identification of the sulfate transporter SST1 in the symbiosomal membrane, the finding that glutathione produced in the bacteroids and host cells is essential for nodule activity, and the demonstration that sulfur assimilation in the whole plant is reprogrammed during symbiosis. However, many crucial questions still remain and some examples follow. In the first place, it is of paramount importance to elucidate the mechanism by which sulfur deficiency limits SNF. It is unknown why homoglutahione replaces glutathione as a major water-soluble antioxidant, redox buffer, and sulfur reservoir, among other relevant functions, only in certain legumes and also in different tissues of the same legume species. Much more work is required to identify oxidative post-translational modifications entailing cysteine and methionine residues and to determine how these modifications affect protein function and metabolism in nodules. Likewise, most interactions of antioxidant metabolites and enzymes bearing redox-active sulfur with transcription factors need to be defined. Solving these questions will pave the way to decipher sulfur-dependent mechanisms that regulate SNF, thereby gaining a deep insight into how nodulated legumes adapt to the fluctuating availability of nutrients in the soil.
Keywords: (homo)glutathione; bacteroids; cysteine; legume nodules; sulfur metabolism; symbiosis.
Figures

References
-
- Abbas B. A., Vineetha K. E., Prasad C. K., Vij N., Hassani R., Randhawa G. S. (2002). Symbiotic characteristics of cysteine and methionine auxotrophs of Sinorhizobium meliloti. Indian J. Exp. Bot. 40 1121–1130. - PubMed
-
- Anderson A. J., Spencer D. (1950). Sulphur in nitrogen metabolism of legumes and non-legumes. Aust. J. Sci. Res. 3 414–430. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

