Altered Cd8+ T lymphocyte Response Triggered by Arginase 1: Implication for Fatigue Intensification during Localized Radiation Therapy in Prostate Cancer Patients
- PMID: 30364895
- PMCID: PMC6198670
- DOI: 10.4172/Neuropsychiatry.1000454
Altered Cd8+ T lymphocyte Response Triggered by Arginase 1: Implication for Fatigue Intensification during Localized Radiation Therapy in Prostate Cancer Patients
Abstract
Fatigue, the most common side effect of cancer treatments, is observed to intensify during external-beam radiation therapy (EBRT). The underlying molecular mechanisms remain unclear. This study investigated the differentially expressed genes/proteins and their association with fatigue intensification during EBRT. Fatigue scores measured by FACT-F and peripheral blood were collected prior to treatment (baseline D0), at midpoint (days 19-21, D21) and endpoint (days 38-42, D42) from men (n=30) with non-metastatic prostate cancer undergoing EBRT. RNA extracted from peripheral blood was used for gene expression analysis. Plasma arginase I and arginine were examined using ELISA and liquid chromatography-tandem mass spectrometry. Differences in fatigue scores, gene and protein expression between times points following EBRT were analyzed by one way ANOVA followed by Post Hoc t-test. Fatigue scores decreased significantly from baseline (44.6 ± 8.1) to midpoint (37.3 ± 10.6, p=0.000, low scores indicating high fatigue) and to endpoint (37.4 ± 10.1, p=0.001) during EBRT. ARG1 (encoding arginase type 1) was significantly up regulated from baseline to midpoint of EBRT (fold change =2.41, p<0.05) whereas genes associated with the adaptive immune functional pathway (CD28, CD27, CCR7, CD3D, CD8A and HLA-DOB) were significantly downregulated >2-fold between the study time points. The changes in gene expression were associated with patient reported fatigue intensity. Moreover, the upregulation of ARG1 was negatively correlated with the absolute lymphocyte count (R2=0.561, p=0.01) only in the high level of fatigue group (n=17) during EBRT. Increased ARG1 expression is known to result in arginine deficiency, which leads to immunosuppression by impairing lymphocyte proliferation and activation. EBRT-induced ARG1 upregulation may play an essential role in fatigue intensification via the arginine deficiency and suppression of T-cell proliferation pathways. These findings may provide novel insights into the molecular-genetic mechanisms underlying the development and intensification of cancer treatment-related fatigue.
Keywords: Cancer-related fatigue (CRF); External beam radiation therapy (EBRT); Gene expression; Lymphocyte; Prostate cancer.
Figures
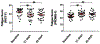
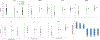


Comment in
-
Cancer related fatigue in prostate cancer.Transl Androl Urol. 2019 Mar;8(Suppl 1):S106-S108. doi: 10.21037/tau.2018.12.06. Transl Androl Urol. 2019. PMID: 31143682 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
