Non-invasive tracking of disease progression in young dystrophic muscles using multi-parametric MRI at 14T
- PMID: 30365532
- PMCID: PMC6203357
- DOI: 10.1371/journal.pone.0206323
Non-invasive tracking of disease progression in young dystrophic muscles using multi-parametric MRI at 14T
Abstract
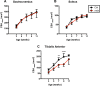
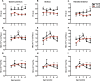
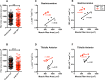
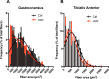
In this study, multi-parametric magnetic resonance imaging (MRI) was conducted to monitor skeletal muscle changes in dystrophic (mdx4cv) and age-matched control (C57BL/6J) mice starting at 3 weeks of age. The objective of this study was to evaluate and characterize changes in muscle tissue characteristics of hind limbs in young, dystrophic mice using MRI. Mdx4cv (n = 25) and age-matched C57BL/6J (n = 5) were imaged at 3, 5, 7, 9, and 11 weeks of age. Multiple MR measurements were taken from the tibialis anterior, gastrocnemius, and soleus muscles. There were significant differences between dystrophic and control groups for all three muscle types when comparing transverse relaxation times (T2) in lower hind limb muscles. Additionally, fractional anisotropy, radial diffusivity, and eigenvalue analysis of diffusion tensor imaging also demonstrated significant differences between groups. Longitudinal relaxation times (T1) displayed no significant differences between groups. The earliest time points in the magnetization transfer ratio measurements displayed a significant difference. Histological analysis revealed significant differences in the tibialis anterior and gastrocnemius muscles between groups with the mdx mice displaying greater variability in muscle fiber size in later time points. The multi-parametric MRI approach offers a promising alternative for future development of a noninvasive avenue for tracking both disease progression and treatment response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

