The Citrobacter rodentium type III secretion system effector EspO affects mucosal damage repair and antimicrobial responses
- PMID: 30365535
- PMCID: PMC6221368
- DOI: 10.1371/journal.ppat.1007406
The Citrobacter rodentium type III secretion system effector EspO affects mucosal damage repair and antimicrobial responses
Abstract
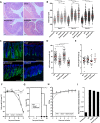
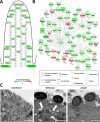
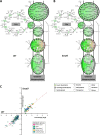
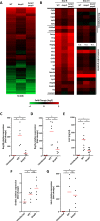
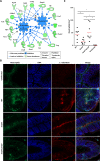
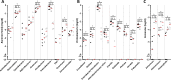
Infection with Citrobacter rodentium triggers robust tissue damage repair responses, manifested by secretion of IL-22, in the absence of which mice succumbed to the infection. Of the main hallmarks of C. rodentium infection are colonic crypt hyperplasia (CCH) and dysbiosis. In order to colonize the host and compete with the gut microbiota, C. rodentium employs a type III secretion system (T3SS) that injects effectors into colonic intestinal epithelial cells (IECs). Once injected, the effectors subvert processes involved in innate immune responses, cellular metabolism and oxygenation of the mucosa. Importantly, the identity of the effector/s triggering the tissue repair response is/are unknown. Here we report that the effector EspO ,an orthologue of OspE found in Shigella spp, affects proliferation of IECs 8 and 14 days post C. rodentium infection as well as secretion of IL-22 from colonic explants. While we observed no differences in the recruitment of group 3 innate lymphoid cells (ILC3s) and T cells, which are the main sources of IL-22 at the early and late stages of C. rodentium infection respectively, infection with ΔespO was characterized by diminished recruitment of sub-mucosal neutrophils, which coincided with lower abundance of Mmp9 and chemokines (e.g. S100a8/9) in IECs. Moreover, mice infected with ΔespO triggered significantly lesser nutritional immunity (e.g. calprotectin, Lcn2) and expression of antimicrobial peptides (Reg3β, Reg3γ) compared to mice infected with WT C. rodentium. This overlapped with a decrease in STAT3 phosphorylation in IECs. Importantly, while the reduced CCH and abundance of antimicrobial proteins during ΔespO infection did not affect C. rodentium colonization or the composition of commensal Proteobacteria, they had a subtle consequence on Firmicutes subpopulations. EspO is the first bacterial virulence factor that affects neutrophil recruitment and secretion of IL-22, as well as expression of antimicrobial and nutritional immunity proteins in IECs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Barthold SW (1980) The microbiology of transmissible murine colonic hyperplasia. Lab Anim Sci 30: 167–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

