Zinc-dependent substrate-level phosphorylation powers Salmonella growth under nitrosative stress of the innate host response
- PMID: 30365536
- PMCID: PMC6221366
- DOI: 10.1371/journal.ppat.1007388
Zinc-dependent substrate-level phosphorylation powers Salmonella growth under nitrosative stress of the innate host response
Abstract
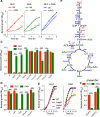
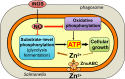
The metabolic processes that enable the replication of intracellular Salmonella under nitrosative stress conditions engendered in the innate response of macrophages are poorly understood. A screen of Salmonella transposon mutants identified the ABC-type high-affinity zinc uptake system ZnuABC as a critical determinant of the adaptation of Salmonella to the nitrosative stress generated by the enzymatic activity of inducible nitric oxide (NO) synthase of mononuclear phagocytic cells. NO limits the virulence of a znuB mutant in an acute murine model of salmonellosis. The ZnuABC transporter is crucial for the glycolytic function of fructose bisphosphate aldolase, thereby fueling growth of Salmonella during nitrosative stress produced in the innate response of macrophages. Our investigations demonstrate that glycolysis mediates resistance of Salmonella to the antimicrobial activity of NO produced in an acute model of infection. The ATP synthesized by substrate-level phosphorylation at the payoff phase of glycolysis and acetate fermentation powers the replication of Salmonella experiencing high levels of nitrosative stress. In contrast, despite its high potential for ATP synthesis, oxidative phosphorylation is a major target of inhibition by NO and contributes little to the antinitrosative defenses of intracellular Salmonella. Our investigations have uncovered a previously unsuspected conjunction between zinc homeostasis, glucose metabolism and cellular energetics in the adaptation of intracellular Salmonella to the reactive nitrogen species synthesized in the innate host response.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Codependent and independent effects of nitric oxide-mediated suppression of PhoPQ and Salmonella pathogenicity island 2 on intracellular Salmonella enterica serovar typhimurium survival.Infect Immun. 2009 Nov;77(11):5107-15. doi: 10.1128/IAI.00759-09. Epub 2009 Sep 8. Infect Immun. 2009. PMID: 19737903 Free PMC article.
-
Cytochrome bd-Dependent Bioenergetics and Antinitrosative Defenses in Salmonella Pathogenesis.mBio. 2016 Dec 20;7(6):e02052-16. doi: 10.1128/mBio.02052-16. mBio. 2016. PMID: 27999164 Free PMC article.
-
DksA-dependent resistance of Salmonella enterica serovar Typhimurium against the antimicrobial activity of inducible nitric oxide synthase.Infect Immun. 2012 Apr;80(4):1373-80. doi: 10.1128/IAI.06316-11. Epub 2012 Feb 6. Infect Immun. 2012. PMID: 22311927 Free PMC article.
-
Oxygen-dependent anti-Salmonella activity of macrophages.Trends Microbiol. 2001 Jan;9(1):29-33. doi: 10.1016/s0966-842x(00)01897-7. Trends Microbiol. 2001. PMID: 11166240 Review.
-
Inducible nitric oxide synthase and Salmonella infection.Microbes Infect. 2001 Jul;3(9):771-6. doi: 10.1016/s1286-4579(01)01428-9. Microbes Infect. 2001. PMID: 11489426 Review.
Cited by
-
Itaconate is an effector of a Rab GTPase cell-autonomous host defense pathway against Salmonella.Science. 2020 Jul 24;369(6502):450-455. doi: 10.1126/science.aaz1333. Science. 2020. PMID: 32703879 Free PMC article.
-
The zinc transporter Slc30a1 (ZnT1) in macrophages plays a protective role against attenuated Salmonella.Elife. 2024 Oct 30;13:e89509. doi: 10.7554/eLife.89509. Elife. 2024. PMID: 39475776 Free PMC article.
-
Who's in control? Regulation of metabolism and pathogenesis in space and time.Curr Opin Microbiol. 2020 Jun;55:88-96. doi: 10.1016/j.mib.2020.05.009. Epub 2020 Jun 9. Curr Opin Microbiol. 2020. PMID: 32532689 Free PMC article. Review.
-
Anaerobic respiration of host-derived methionine sulfoxide protects intracellular Salmonella from the phagocyte NADPH oxidase.Cell Host Microbe. 2024 Mar 13;32(3):411-424.e10. doi: 10.1016/j.chom.2024.01.004. Epub 2024 Feb 1. Cell Host Microbe. 2024. PMID: 38307020 Free PMC article.
-
Glycolytic reprograming in Salmonella counters NOX2-mediated dissipation of ΔpH.Nat Commun. 2020 Apr 14;11(1):1783. doi: 10.1038/s41467-020-15604-2. Nat Commun. 2020. PMID: 32286292 Free PMC article.
References
-
- Alvarez-Ordonez A, Prieto M, Bernardo A, Hill C, Lopez M. The Acid Tolerance Response of Salmonella spp.: An adaptive strategy to survive in stressful environments prevailing in foods and the host. Food Research International. 2012;45(2):482–92. 10.1016/j.foodres.2011.04.002 WOS:000302032200003. - DOI

