Characterization and implications of the dynamics of eosinophils in blood and in the infarcted myocardium after coronary reperfusion
- PMID: 30365558
- PMCID: PMC6203260
- DOI: 10.1371/journal.pone.0206344
Characterization and implications of the dynamics of eosinophils in blood and in the infarcted myocardium after coronary reperfusion
Abstract
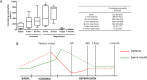
Objective: We characterized the dynamics of eosinophils in blood and in the infarcted myocardium in patients and in a swine model of reperfused myocardial infarction (MI). The association of eosinophil dynamics with various outcomes was assessed.
Methods: Serial eosinophil count and pre-discharge cardiac magnetic resonance were carried out in a prospective series of 620 patients with a first ST-elevation MI. In a swine model of reperfused MI, the dynamics of circulating eosinophils and their presence in the infarcted myocardium were determined. In autopsies from chronic MI patients, eosinophils were quantified.
Results: Patient eosinophil count sharply decreased 12h post-reperfusion compared to arrival. A lower minimum eosinophil count was associated with more extensive edema, microvascular obstruction, and infarct size as measured by cardiac magnetic resonance, and also with a higher rate of cardiac events (death, re-infarction, or heart failure) during follow-up. In the experimental model, eosinophil count boosted during ischemia and dropped back immediately post-reperfusion. Myocardial samples revealed progressive eosinophil migration into the infarcted myocardium, especially areas with microvascular obstruction. Markers of eosinophil maturation and survival (interleukin-5), degranulation (eosinophil cationic protein) and migration (eotoxin-1) were detected in the blood of patients, and in porcine myocardium. Eosinophil infiltration was detected in autopsies from chronic MI patients.
Conclusion: Eosinopenia post-MI was associated with an impaired cardiac structure and adverse events. The decay in circulating eosinophils soon after reperfusion mirrors their migration into the infarcted myocardium, as reflected by their presence in heart samples from swine and patients. Further studies are needed to understanding this unexplored pathway and its therapeutic implications.
Conflict of interest statement
The authors have declared no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

