New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives
- PMID: 30366133
- PMCID: PMC6291345
- DOI: 10.1016/j.actbio.2018.10.033
New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives
Abstract
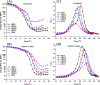
Resin-based composite has overtaken dental amalgam as the most popular material for direct restorative dentistry. In spite of this popularity the clinical lifetime of composite restorations is threatened by recurrent decay. Degradation of the adhesive leads to gaps at the composite/tooth interface-bacteria, bacterial by-products and fluids infiltrate the gaps leading to recurrent decay and composite restoration failure. The durability of resin-dentin bonds is a major problem. We address this problem by synthesizing silyl-functionalized BisGMA (e.g., silyl-BisGMA), formulating dental adhesives with the new monomer and determining the physicochemical properties and leaching characteristics of the silyl-BisGMA adhesives. Silyl-BisGMA was synthesized by stoichiometric amounts of BisGMA and 3-isocyanatopropyl trimethoxysilane (IPTMS). The control adhesive was a mixture based on HEMA/BisGMA (45/55, w/w). In the experimental formulations, BisGMA was partially or completely replaced by silyl-BisGMA. Water miscibility, polymerization behavior (Fourier transform infrared spectroscopy, FTIR), thermal property (modulated differential scanning calorimetry, MDSC), mechanical properties in dry and wet conditions (dynamic mechanical analysis, DMA), and leached species (HPLC) were investigated. Data from all tests were submitted to appropriate statistical analysis (α = 0.05). Silyl-BisGMA-containing adhesives exhibited comparable water miscibility, lower viscosities, and significantly improved degree of conversion of CC bond as compared to the control. After 4 weeks aqueous aging, the glass transition temperature and rubbery moduli of the experimental copolymers were significantly greater than the control (p < 0.05). HPLC results indicated a substantial reduction of leached HEMA (up to 99 wt%) and BisGMA (up to 90 wt%). By introducing silyl-functional group, the new BisGMA derivative exhibited potential as a monomer that can lead to dental adhesives with improved mechanical properties and reduced leaching under conditions relevant to the oral environment. STATEMENT OF SIGNIFICANCE: The low-viscosity adhesive that bonds the composite to the tooth (enamel and dentin) is intended to seal and stabilize the composite/tooth interface, but it degrades leading to a breach at the composite/tooth margin. As the most popular crosslinking monomer in adhesives, Bisphenol A-glycerolate dimethacrylate (BisGMA) has limitations, e.g. susceptible to hydrolysis and concomitant property degradation. A methoxysilyl-functionalized BisGMA derivative (silyl-BisGMA) was introduced in this work to respond to these limitations. Our results indicated that by introducing silyl-BisGMA, higher crosslinked networks were obtained without sacrificing the homogeneity, and the leached amount of HEMA was reduced up to 99%. This novel resin offers potential benefits including prolonging the functional lifetime of dental resin materials.
Keywords: BisGMA; Dental adhesive; Dynamic mechanical analysis; Elution; Polymerization kinetic; Self-strengthening.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures





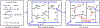
References
-
- Nedeljkovic I, Teughels W, De Munck J, Van Meerbeek B, Van Landuyt KL, Is secondary caries with composites a material-based problem?, Dent. Mater, 31 (2015) E247–E277. - PubMed
-
- Manuja N, Nagpal R, Pandit IK, Dental Adhesion: Mechanism, Techniques and Durability, J. Clin. Pediatr. Dent, 36 (2012) 223–234. - PubMed
-
- El-Deeb HA, Al Sherbiney HH, Mobarak EH, Bond Durability of Adhesives Containing Modified-monomer With/Without-fluoride After Aging in Artificial Saliva and Under Intrapulpal Pressure Simulation, Oper. Dent, 38 (2013) 48–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

