Use of non-invasive measures to predict cochlear synapse counts
- PMID: 30366194
- PMCID: PMC7161203
- DOI: 10.1016/j.heares.2018.10.006
Use of non-invasive measures to predict cochlear synapse counts
Abstract
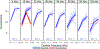
Cochlear synaptopathy, the loss of synaptic connections between inner hair cells (IHCs) and auditory nerve fibers, has been documented in animal models of aging, noise, and ototoxic drug exposure, three common causes of acquired sensorineural hearing loss in humans. In each of these models, synaptopathy begins prior to changes in threshold sensitivity or loss of hair cells; thus, this underlying injury can be hidden behind a normal threshold audiogram. Since cochlear synaptic loss cannot be directly confirmed in living humans, non-invasive assays will be required for diagnosis. In animals with normal auditory thresholds, the amplitude of wave 1 of the auditory brainstem response (ABR) is highly correlated with synapse counts. However, synaptopathy can also co-occur with threshold elevation, complicating the use of the ABR alone as a diagnostic measure. Using an age-graded series of mice and a partial least squares regression approach to model structure-function relationships, this study shows that the combination of a small number of ABR and distortion product otoacoustic emission (DPOAE) measurements can predict synaptic ribbon counts at various cochlear frequencies to within 1-2 synapses per IHC of their true value. In contrast, the model, trained using the age-graded series of mice, overpredicted synapse counts in a small sample of young noise-exposed mice, perhaps due to differences in the underlying pattern of damage between aging and noise-exposed mice. These results provide partial validation of a noninvasive approach to identify synaptic/neuronal loss in humans using ABRs and DPOAEs.
Keywords: Auditory brainstem response; Cochlear synaptopathy; Hidden hearing loss; Least-squares analysis; Noise-induced hearing loss; Otoacoustic emissions.
Published by Elsevier B.V.
Conflict of interest statement
Declarations of interest
None.
Figures
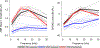

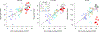
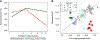
References
-
- Bourien J, Tang Y, Batrel C, et al. (2014). Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol, 112, 1025–1039. - PubMed
-
- Chen MA, Webster P, Yang E, et al. (2006). Presbycusic neuritic degeneration within the osseous spiral lamina. Otology & Neurotology, 27, 316–322. - PubMed
-
- Crowe SJ, Guild SR, Polvogt LM (1934). Observations on the pathology of high-tone deafness. The Journal of Nervous and Mental Disease, 80, 480.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

