Quantitative measurement of T2, T1ρ and T1 relaxation times in articular cartilage and cartilage-bone interface by SE and UTE imaging at microscopic resolution
- PMID: 30366222
- PMCID: PMC6289866
- DOI: 10.1016/j.jmr.2018.10.008
Quantitative measurement of T2, T1ρ and T1 relaxation times in articular cartilage and cartilage-bone interface by SE and UTE imaging at microscopic resolution
Abstract
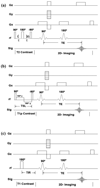
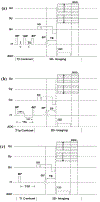
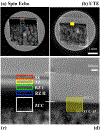
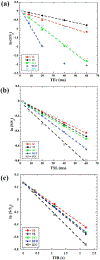
Both spin-echo (SE) and ultra-short echo (UTE) based MRI sequences were used on a 7 T µMRI system to quantify T2, T1ρ and T1 relaxation times from articular cartilage to the cartilage-bone interface on canine humeral specimens at 19.5 µm pixel resolution. A series of five relaxation-weighted images were acquired to calculate one relaxation map (T2, T1ρ or T1), from which the depth-dependent profiles were examined between the SE method and the UTE method, over the entire non-calcified cartilage and within the cartilage-bone interface. SE-based methods enabled the quantification of relaxation profiles over the noncalcified cartilage, from 0 µm (articular surface) to approximately 460 µm in depth (near the end of radial zone). Most of the cartilage-bone interface was imaged by the UTE-based methods, to a tissue depth of about 810 µm. Pixel-by-pixel calculation of the relaxation times between the independent SE and UTE methods correlated well with each other. A better understanding of the tissue properties reliably over the cartilage-bone interface region by a non-invasive MRI approach could contribute to the clinical diagnostics of trauma-induced osteoarthritis.
Keywords: Articular cartilage; Microscopic resolution; Quantitative MRI; T1; T1ρ; T2; UTE; Zone of calcified cartilage.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Maroudas A, Physicochemical properties of articular cartilage, in: Freeman MAR (Ed.) Adult articular cartilage, 2nd ed, Pitman Medical, Kent, England, 1979, pp. 215–290.
-
- Buckwalter J, Mankin H, Articular Cartilage Repair and transplantation, Arthritis & Rheumatism, 41 (1998) 1331–1341. - PubMed
-
- Bullough PG, Yawitz PS, Tafra L, Boskey AL, Topographical variations in the morphology and biochemistry of adult canine tibial plateau articular cartilage, J Orthop Res, 3 (1985) 1–16. - PubMed
-
- Hoemann CD, Lafantaisie-Favreau CH, Lascau-Coman V, Chen G, Guzman-Morales J, The cartilage-bone interface, The journal of knee surgery, 25 (2012) 85–97. - PubMed
-
- Redler I, Mow VC, Zimny ML, Mansell J, The Ultrastructure and Biomechanical Significance of the Tidemark of Articular Cartilage, Clin. Orthop. and Related Topics, 112 (1975) 357–367. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

