Superresolution microscopy with novel BODIPY-based fluorophores
- PMID: 30366346
- PMCID: PMC6203453
- DOI: 10.1371/journal.pone.0206104
Superresolution microscopy with novel BODIPY-based fluorophores
Abstract
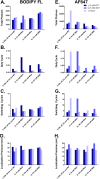
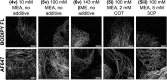
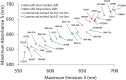
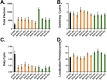
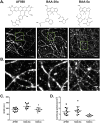
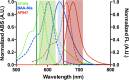
Multicolor single-molecule localization microscopy (SMLM) expands our understanding of subcellular details and enables the study of biomolecular interactions through precise visualization of multiple molecules in a single sample with resolution of ~10-20 nm. Probe selection is vital to multicolor SMLM, as the fluorophores must not only exhibit minimal spectral crosstalk, but also be compatible with the same photochemical conditions that promote fluorophore photoswitching. While there are numerous commercially available photoswitchable fluorophores that are optimally excited in the standard Cy3 channel, they are restricted to short Stokes shifts (<30 nm), limiting the number of colors that can be resolved in a single sample. Furthermore, while imaging buffers have been thoroughly examined for commonly used fluorophore scaffolds including cyanine, rhodamine, and oxazine, optimal conditions have not been found for the BODIPY scaffold, precluding its routine use for multicolor SMLM. Herein, we screened common imaging buffer conditions including seven redox reagents with five additives, resulting in 35 overall imaging buffer conditions to identify compatible combinations for BODIPY-based fluorophores. We then demonstrated that novel, photoswitchable BODIPY-based fluorophores with varied length Stokes shifts provide additional color options for SMLM using a combination of BODIPY-based and commercially available photoswitchable fluorophores.
Conflict of interest statement
Dr. Nick Dolman is an employee of Thermo Fisher Scientific. Participation in this work does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

