Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell
- PMID: 30366368
- PMCID: PMC6315946
- DOI: 10.3390/membranes8040099
Effects of Polymer Matrices and Carbon Nanotubes on the Generation of Electric Energy in a Microbial Fuel Cell
Abstract
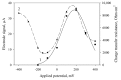
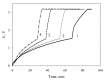
The anode of a microbial fuel cell (MFC) was formed on a graphite electrode and immobilized Gluconobacter oxydans VKM-1280 bacterial cells. Immobilization was performed in chitosan, poly(vinyl alcohol) or N-vinylpyrrolidone-modified poly(vinyl alcohol). Ethanol was used as substrate. The anode was modified using multiwalled carbon nanotubes. The aim of the modification was to create a conductive network between cell lipid membranes, containing exposed pyrroloquinoline quinone (PQQ)-dependent alcoholdehydrogenases, and the electrode to facilitate electron transfer in the system. The bioelectrochemical characteristics of modified anodes at various cell/polymer ratios were assessed via current density, power density, polarization curves and impedance spectres. Microbial fuel cells based on chitosan at a matrix/cell volume ratio of 5:1 produced maximal power characteristics of the system (8.3 μW/cm²) at a minimal resistance (1111 Ohm cm²). Modification of the anode by multiwalled carbon nanotubes (MWCNT) led to a slight decrease of internal resistance (down to 1078 Ohm cm²) and to an increase of generated power density up to 10.6 μW/cm². We explored the possibility of accumulating electric energy from an MFC on a 6800-μF capacitor via a boost converter. Generated voltage was increased from 0.3 V up to 3.2 V. Accumulated energy was used to power a Clark-type biosensor and a Bluetooth transmitter with three sensors, a miniature electric motor and a light-emitting diode.
Keywords: boost converter accumulation; immobilization of bacterial cells; interaction of cell membranes with carbon nanotubes; microbial fuel cell; polymer matrix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nikovskaya G.N. The adhesive immobilization of microorganisms in water purification. Khim. Tekhnol. Vody. 1989;11:158–169.
-
- Kozlyak E.I., Solomon Z.G., Yakimov M.M. The sorption of Pseudomonas fluorescens 16n2 cells on cellulose triacetate fibers. Prikl. Biokhim. Mikrobiol. 1991;27:508–513. - PubMed
-
- Kozlyak E.I., Solomon Z.G., Yakimov M.M., Fadyushina T.V. The sorption of Pseudomonas fluorescens 16n2 cells on various adsorbents. Prikl. Biokhim. Mikrobiol. 1993;29:138–143.
-
- Górecka E., Jastrzębska M. Immobilization techniques and biopolymer carriers. Biotechnol. Food Sci. 2011;75:65–86.
-
- Teles F.R.R., Fonseca L.P. Applications of polymers for biomolecule immobilization in electrochemical biosensors. Mater. Sci. Eng. C. 2008;28:1530–1533. doi: 10.1016/j.msec.2008.04.010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

