Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo
- PMID: 30366383
- PMCID: PMC6274788
- DOI: 10.3390/ijms19113319
Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo
Abstract
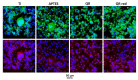
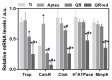
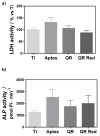
In this study, the effect on osteoclast activity in vitro and in vivo of titanium implants that were coated with quercitrin was evaluated. Titanium surfaces were covalently coated with the flavonoid quercitrin. The effect of the surfaces on osteoclastogenesis was first tested in vitro on RAW264.7 cells that were supplemented with receptor activator of nuclear factor kappa-B ligand (RANKL) to generate osteoclast-like cells by tartrate-resistant acid phosphatase (TRAP) inmunostaining after five days of culture, and by analysis of the mRNA expression levels of markers related to bone resorption after seven days of culture. A rabbit tibial model was used to evaluate the in vivo biological response to the implant surfaces after eight weeks of healing, analyzing the lactate dehydrogenase (LDH) and the alkaline phosphatase (ALP) activities in the wound fluid that were present at the implant interface and the peri-implant bone mRNA expression levels of several markers related to inflammation, bone resorption and osteoblast-osteoclast interaction. No differences between groups and control surfaces were found in the wound fluid analyses. Moreover, quercitrin implant surfaces significantly decreased the expression of osteoclast related genes in vitro (Trap, CalcR, Ctsk, H⁺ATPase, Mmp9) and in vivo (Ctsk, H⁺ATPase, Mmp9) as well as the expression of RankL in vivo. Moreover, quercitrin surfaces were not cytotoxic for the cells. Thus, quercitrin implant surfaces were biocompatible and decreased osteoclastogenesis in vitro and in vivo. This could be used to improve the performance of dental implants.
Keywords: animal experiments; biomaterials; bone implant interactions; polyphenols; surface chemistry.
Conflict of interest statement
We declare the following conflicts of interest: A.C., M.M. and J.M.R. are inventors of a pending patent application based on some aspects of this work (PCT/EP2013/058116). The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures


References
-
- Moraschini V., Poubel L.A., Ferreira D.C., Barboza V.F., Barboza Edos S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015;44:377–388. doi: 10.1016/j.ijom.2014.10.023. - DOI - PubMed
-
- Bauer S., Schmuki P., von der Mark K., Park J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2013;58:261–326. doi: 10.1016/j.pmatsci.2012.09.001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

