Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy
- PMID: 30366391
- PMCID: PMC6278569
- DOI: 10.3390/molecules23112763
Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy
Abstract
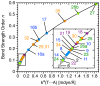
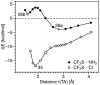
A set of 35 representative neutral and charged tetrel complexes was investigated with the objective of finding the factors that influence the strength of tetrel bonding involving single bonded C, Si, and Ge donors and double bonded C or Si donors. For the first time, we introduced an intrinsic bond strength measure for tetrel bonding, derived from calculated vibrational spectroscopy data obtained at the CCSD(T)/aug-cc-pVTZ level of theory and used this measure to rationalize and order the tetrel bonds. Our study revealed that the strength of tetrel bonds is affected by several factors, such as the magnitude of the σ-hole in the tetrel atom, the negative electrostatic potential at the lone pair of the tetrel-acceptor, the positive charge at the peripheral hydrogen of the tetrel-donor, the exchange-repulsion between the lone pair orbitals of the peripheral atoms of the tetrel-donor and the heteroatom of the tetrel-acceptor, and the stabilization brought about by electron delocalization. Thus, focusing on just one or two of these factors, in particular, the σ-hole description can only lead to an incomplete picture. Tetrel bonding covers a range of -1.4 to -26 kcal/mol, which can be strengthened by substituting the peripheral ligands with electron-withdrawing substituents and by positively charged tetrel-donors or negatively charged tetrel-acceptors.
Keywords: CCSD(T); intrinsic bond strength; local stretching force constant; noncovalent interactions; tetrel bonding; weak interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Politzer P., Murray J.S. Analysis of Halogen and Other σ-Hole Bonds in Crystals. Crystals. 2018;8:42. doi: 10.3390/cryst8010042. - DOI
-
- Scheiner S. Hydrogen Bonding: A Theoretical Perspective. Oxford University Press; New York, NY, USA: 1997.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

