Kidney-on-a-chip: untapped opportunities
- PMID: 30366681
- PMCID: PMC6408139
- DOI: 10.1016/j.kint.2018.06.034
Kidney-on-a-chip: untapped opportunities
Abstract
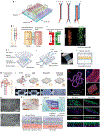
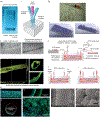
The organs-on-a-chip technology has shown strong promise in mimicking the complexity of native tissues in vitro and ex vivo, and recently significant advances have been made in applying this technology to studies of the kidney and its diseases. Individual components of the nephron, including the glomerulus, proximal tubule, and distal tubule/medullary collecting duct, have been successfully mimicked using organs-on-a-chip technology and yielding strong promises in advancing the field of ex vivo drug toxicity testing and augmenting renal replacement therapies. Although these models show promise over 2-dimensional cell systems in recapitulating important nephron features in vitro, nephron functions, such as tubular secretion, intracellular metabolism, and renin and vitamin D production, as well as prostaglandin synthesis are still poorly recapitulated in on-chip models. Moreover, construction of multiple-renal-components-on-a-chip models, in which various structures and cells of the renal system interact with each other, has remained a challenge. Overall, on-chip models show promise in advancing models of normal and pathological renal physiology, in predicting nephrotoxicity, and in advancing treatment of chronic kidney diseases.
Keywords: kidney; microfluidics; microphysiological systems; organ-on-a-chip; tissue engineering.
Published by Elsevier Inc.
Figures

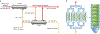
References
-
- Sanechika N, Sawada K, Usui Y, et al. Development of bioartificial renal tubule devices with lifespan-extended human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2011;26:2761–2769. - PubMed
-
- Mackay S, Funke A, Buffington D, Humes H. Tissue engineering of a bioartificial renal tubule. ASAIO J. 1998;44:179–183. - PubMed
-
- Jang K, Suh K. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36–42. - PubMed
-
- Hartung T. Toxicology for the twenty-first century. Nature. 2009;460:208–212. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

