Brain mechanisms underlying apathy
- PMID: 30366958
- PMCID: PMC6518466
- DOI: 10.1136/jnnp-2018-318265
Brain mechanisms underlying apathy
Abstract
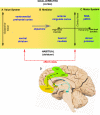
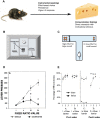
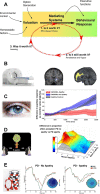
The past few decades have seen growing interest in the neuropsychiatric syndrome of apathy, conceptualised as a loss of motivation manifesting as a reduction of goal-directed behaviour. Apathy occurs frequently, and with substantial impact on quality of life, in a broad range of neurological and psychiatric conditions. Apathy is also consistently associated with neuroimaging changes in specific medial frontal cortex and subcortical structures, suggesting that disruption of a common systems-level mechanism may underlie its development, irrespective of the condition that causes it. In parallel with this growing recognition of the clinical importance of apathy, significant advances have been made in understanding normal motivated behaviour in humans and animals. These developments have occurred at several different conceptual levels, from work linking neural structures and neuromodulatory systems to specific aspects of motivated behaviour, to higher order computational models that aim to unite these findings within frameworks for normal goal-directed behaviour. In this review we develop a conceptual framework for understanding pathological apathy based on this current understanding of normal motivated behaviour. We first introduce prominent theories of motivated behaviour-which often involves sequences of actions towards a goal that needs to be maintained across time. Next, we outline the behavioural effects of disrupting these processes in animal models, highlighting the specific effects of these manipulations on different components of motivated behaviour. Finally, we relate these findings to clinical apathy, demonstrating the homologies between this basic neuroscience work and emerging behavioural and physiological evidence from patient studies of this syndrome.
Keywords: apathy; cognitive neuroscience; decision making; effort; goal-directed behaviour; motivation; reward.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
