Siderophore-Mediated Iron Acquisition Enhances Resistance to Oxidative and Aromatic Compound Stress in Cupriavidus necator JMP134
- PMID: 30366993
- PMCID: PMC6293101
- DOI: 10.1128/AEM.01938-18
Siderophore-Mediated Iron Acquisition Enhances Resistance to Oxidative and Aromatic Compound Stress in Cupriavidus necator JMP134
Abstract
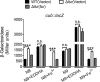
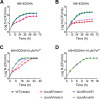
Many bacteria secrete siderophores to enhance iron uptake under iron-restricted conditions. In this study, we found that Cupriavidus necator JMP134, a well-known aromatic pollutant-degrading bacterium, produces an unknown carboxylate-type siderophore named cupriabactin to overcome iron limitation. Using genome mining, targeted mutagenesis, and biochemical analysis, we discovered an operon containing six open reading frames (cubA-F) in the C. necator JMP134 genome that encodes proteins required for the biosynthesis and uptake of cupriabactin. As the dominant siderophore of C. necator JMP134, cupriabactin promotes the growth of C. necator JMP134 under iron-limited conditions via enhanced ferric iron uptake. Furthermore, we demonstrated that the iron concentration-dependent expression of the cub operon is mediated by the ferric uptake regulator (Fur). Physiological analyses revealed that the cupriabactin-mediated iron acquisition system influences swimming motility, biofilm formation, and resistance to oxidative and aromatic compound stress in C. necator JMP134. In conclusion, we identified a carboxylate-type siderophore named cupriabactin, which plays important roles in iron scavenging, bacterial motility, biofilm formation, and stress resistance.IMPORTANCE Since siderophores have been widely exploited for agricultural, environmental, and medical applications, the identification and characterization of new siderophores from different habitats and organisms will have great beneficial applications. Here, we identified a novel siderophore-producing gene cluster in C. necator JMP134. This gene cluster produces a previously unknown carboxylate siderophore, cupriabactin. Physiological analyses revealed that the cupriabactin-mediated iron acquisition system influences swimming motility, biofilm formation, and oxidative stress resistance. Most notably, this system also plays important roles in increasing the resistance of C. necator JMP134 to stress caused by aromatic compounds, which provide a promising strategy to engineer more efficient approaches to degrade aromatic pollutants.
Keywords: Cupriavidus necator; Fur; aromatic compound degradation; biofilm; oxidative stress; siderophore.
Copyright © 2018 American Society for Microbiology.
Figures







References
-
- Tanabe T, Naka A, Aso H, Nakao H, Narimatsu S, Inoue Y, Ono T, Yamamoto S. 2005. A novel aerobactin utilization cluster in Vibrio vulnificus with a gene involved in the transcription regulation of the iutA homologue. Microbiol Immunol 49:823–834. doi: 10.1111/j.1348-0421.2005.tb03671.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

