Lsm12 Mediates Deubiquitination of DNA Polymerase η To Help Saccharomyces cerevisiae Resist Oxidative Stress
- PMID: 30366994
- PMCID: PMC6293111
- DOI: 10.1128/AEM.01988-18
Lsm12 Mediates Deubiquitination of DNA Polymerase η To Help Saccharomyces cerevisiae Resist Oxidative Stress
Abstract
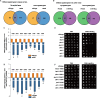
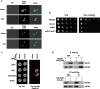
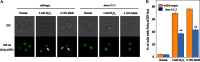
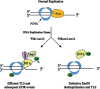
In Saccharomyces cerevisiae, the Y family DNA polymerase η (Polη) regulates genome stability in response to different forms of environmental stress by translesion DNA synthesis. To elucidate the role of Polη in oxidative stress-induced DNA damage, we deleted or overexpressed the corresponding gene RAD30 and used transcriptome analysis to screen the potential genes associated with RAD30 to respond to DNA damage. Under 2 mM H2O2 treatment, the deletion of RAD30 resulted in a 2.2-fold decrease in survival and a 2.8-fold increase in DNA damage, whereas overexpression of RAD30 increased survival and decreased DNA damage by 1.2- and 1.4-fold, respectively, compared with the wild-type strain. Transcriptome and phenotypic analyses identified Lsm12 as a main factor involved in oxidative stress-induced DNA damage. Deleting LSM12 caused growth defects, while its overexpression enhanced cell growth under 2 mM H2O2 treatment. This effect was due to the physical interaction of Lsm12 with the UBZ domain of Polη to enhance Polη deubiquitination through Ubp3 and consequently promote Polη recruitment. Overall, these findings demonstrate that Lsm12 is a novel regulator mediating Polη deubiquitination to promote its recruitment under oxidative stress. Furthermore, this study provides a potential strategy to maintain the genome stability of industrial strains during fermentation.IMPORTANCE Polη was shown to be critical for cell growth in the yeast Saccharomyces cerevisiae, and deletion of its corresponding gene RAD30 caused a severe growth defect under exposure to oxidative stress with 2 mM H2O2 Furthermore, we found that Lsm12 physically interacts with Polη and promotes Polη deubiquitination and recruitment. Overall, these findings indicate Lsm12 is a novel regulator mediating Polη deubiquitination that regulates its recruitment in response to DNA damage induced by oxidative stress.
Keywords: DNA damage; Polη; deubiquitination; oxidative stress; recruitment.
Copyright © 2018 American Society for Microbiology.
Figures


Similar articles
-
Sml1 Inhibits the DNA Repair Activity of Rev1 in Saccharomyces cerevisiae during Oxidative Stress.Appl Environ Microbiol. 2020 Mar 18;86(7):e02838-19. doi: 10.1128/AEM.02838-19. Print 2020 Mar 18. Appl Environ Microbiol. 2020. PMID: 32005731 Free PMC article.
-
Role of DNA polymerase eta in the bypass of abasic sites in yeast cells.Nucleic Acids Res. 2004 Jul 29;32(13):3984-94. doi: 10.1093/nar/gkh710. Print 2004. Nucleic Acids Res. 2004. PMID: 15284331 Free PMC article.
-
NEDDylation antagonizes ubiquitination of proliferating cell nuclear antigen and regulates the recruitment of polymerase η in response to oxidative DNA damage.Protein Cell. 2018 Apr;9(4):365-379. doi: 10.1007/s13238-017-0455-x. Epub 2017 Aug 22. Protein Cell. 2018. PMID: 28831681 Free PMC article.
-
Deficiency of Polη in Saccharomyces cerevisiae reveals the impact of transcription on damage-induced cohesion.PLoS Genet. 2021 Sep 9;17(9):e1009763. doi: 10.1371/journal.pgen.1009763. eCollection 2021 Sep. PLoS Genet. 2021. PMID: 34499654 Free PMC article.
-
Genome-Wide Transcriptional Response of Saccharomyces cerevisiae to Stress-Induced Perturbations.Front Bioeng Biotechnol. 2016 Feb 18;4:17. doi: 10.3389/fbioe.2016.00017. eCollection 2016. Front Bioeng Biotechnol. 2016. PMID: 26925399 Free PMC article. Review.
Cited by
-
Sml1 Inhibits the DNA Repair Activity of Rev1 in Saccharomyces cerevisiae during Oxidative Stress.Appl Environ Microbiol. 2020 Mar 18;86(7):e02838-19. doi: 10.1128/AEM.02838-19. Print 2020 Mar 18. Appl Environ Microbiol. 2020. PMID: 32005731 Free PMC article.
-
A New Drug Discovery Platform: Application to DNA Polymerase Eta and Apurinic/Apyrimidinic Endonuclease 1.Int J Mol Sci. 2023 Nov 23;24(23):16637. doi: 10.3390/ijms242316637. Int J Mol Sci. 2023. PMID: 38068959 Free PMC article.
-
Mediator Engineering of Saccharomyces cerevisiae To Improve Multidimensional Stress Tolerance.Appl Environ Microbiol. 2022 Apr 26;88(8):e0162721. doi: 10.1128/aem.01627-21. Epub 2022 Apr 4. Appl Environ Microbiol. 2022. PMID: 35369708 Free PMC article.
-
Identification of RNA-splicing factor Lsm12 as a novel tumor-associated gene and a potent biomarker in Oral Squamous Cell Carcinoma (OSCC).J Exp Clin Cancer Res. 2022 Apr 21;41(1):150. doi: 10.1186/s13046-022-02355-9. J Exp Clin Cancer Res. 2022. PMID: 35449073 Free PMC article.
-
Role of gene regulation and inter species interaction as a key factor in gut microbiota adaptation.Arch Microbiol. 2022 May 20;204(6):342. doi: 10.1007/s00203-022-02935-5. Arch Microbiol. 2022. PMID: 35595857 Review.
References
-
- Saini P, Beniwal A, Vij S. 2017. Physiological response of Kluyveromyces marxianus during oxidative and osmotic stress. Process Biochem 56:21–29. doi:10.1016/j.procbio.2017.03.001. - DOI
-
- Roukas T. 2016. The role of oxidative stress on carotene production by Blakeslea trispora in submerged fermentation. Crit Rev Biotechnol 36:424–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

