Missing elimination via membrane vesicle shedding contributes to the diminished calcium sensitivity of listeriolysin O
- PMID: 30367146
- PMCID: PMC6203718
- DOI: 10.1038/s41598-018-34031-4
Missing elimination via membrane vesicle shedding contributes to the diminished calcium sensitivity of listeriolysin O
Abstract
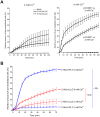
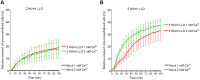
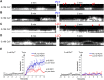
The lytic capacity of cholesterol-dependent cytolysins is enhanced in the extracellular calcium-free environment through a combination of limited membrane repair and diminished membrane toxin removal. For a typical neurotoxin of the group, pneumolysin, this effect has already been observed at reduced (1 mM) calcium conditions, which are pathophysiologically relevant. Here, we tested another neurotoxin of the group, listeriolysin O from L. monocytogenes, active in the primary vacuole after bacterium phagocytosis in host cells. Reduced calcium did not increase the lytic capacity of listeriolysin (in contrast to pneumolysin), while calcium-free conditions elevated it 2.5 times compared to 10 times for pneumolysin (at equivalent hemolytic capacities). To clarify these differences, we analyzed membrane vesicle shedding, known to be a calcium-dependent process for toxin removal from eukaryotic cell membranes. Both pneumolysin and listeriolysin initiated vesicle shedding, which was completely blocked by the lack of extracellular calcium. Lack of calcium, however, elevated the toxin load per a cell only for pneumolysin and not for listeriolysin. This result indicates that vesicle shedding does not play a role in the membrane removal of listeriolysin and outlines a major difference between it and other members of the CDC group. Furthermore, it provides new tools for studying membrane vesicle shedding.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- SFB-TR 84/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- SFB-TR 84/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- IL 151.1-1/Deutsche Forschungsgemeinschaft (German Research Foundation)/International
- WT094762MA/WT_/Wellcome Trust/United Kingdom
- 31003A_160136/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International

