Single-Stranded Nucleic Acids Regulate TLR3/4/7 Activation through Interference with Clathrin-Mediated Endocytosis
- PMID: 30367171
- PMCID: PMC6203749
- DOI: 10.1038/s41598-018-33960-4
Single-Stranded Nucleic Acids Regulate TLR3/4/7 Activation through Interference with Clathrin-Mediated Endocytosis
Abstract
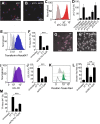
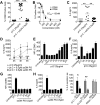
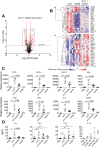
Recognition of nucleic acids by endosomal Toll-like receptors (TLR) is essential to combat pathogens, but requires strict control to limit inflammatory responses. The mechanisms governing this tight regulation are unclear. We found that single-stranded oligonucleotides (ssON) inhibit endocytic pathways used by cargo destined for TLR3/4/7 signaling endosomes. Both ssDNA and ssRNA conferred the endocytic inhibition, it was concentration dependent, and required a certain ssON length. The ssON-mediated inhibition modulated signaling downstream of TLRs that localized within the affected endosomal pathway. We further show that injection of ssON dampens dsRNA-mediated inflammatory responses in the skin of non-human primates. These studies reveal a regulatory role for extracellular ssON in the endocytic uptake of TLR ligands and provide a mechanistic explanation of their immunomodulation. The identified ssON-mediated interference of endocytosis (SOMIE) is a regulatory process that temporarily dampens TLR3/4/7 signaling, thereby averting excessive immune responses.
Conflict of interest statement
P.J., A.D., A.E.S., and AL.S are authors of patent applications related to the work and are shareholders of TIRmed Pharma.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

